NDC Code(s) : 66715-5505-0, 66715-6705-4, 66715-6705-0
Packager : Lil Drug Store Products, Inc
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Circle K Triple Antibioticbacitracin zinc, polymyxin b sulfate, and neomycin sulfate ointment OINTMENT | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Circle K Triple AntibioticBacitracin zinc, Polymyxin B sulfate, and Neomycin sulfate OINTMENT | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Lil Drug Store Products, Inc(093103646) |
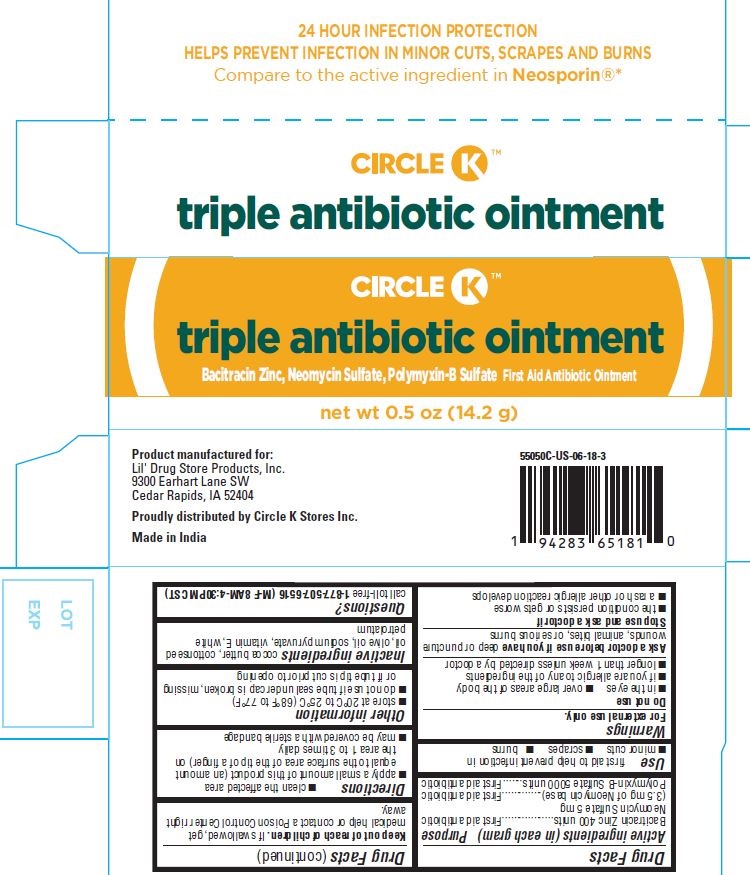
PRINCIPAL DISPLAY PANEL
24 HOUR
Infection Protection
Help Prevent Infection in
Minor Cuts, Scrapes and Burns
CIRCLE K ®
Compare to the Active
Ingredients in Neosporin
®*
Triple Antibiotic Ointment
Bacitracin Zinc, Neomycin Sulfate, Polymyxin-B Sulfate
First Aid Antibiotic Ointment
NET WT 0.5 oz (14.2 g)

PRINCIPAL DISPLAY PANEL
CIRCLE K™
triple antibiotic ointment
Bacitracin Zinc, Neomycin Sulfate, Polymyxin-B Sulfate First Aid Antibiotic Ointment
net wt 0.5 oz (14.2 g)