NDC Code(s) : 66993-086-96, 66993-087-96, 66993-088-96
Packager : Prasco Laboratories
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Fluticasone Propionate and Salmeterol HFAfluticasone propionate and salmeterol xinafoate AEROSOL, METERED | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Fluticasone Propionate and Salmeterol HFAfluticasone propionate and salmeterol xinafoate AEROSOL, METERED | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Fluticasone Propionate and Salmeterol HFAfluticasone propionate and salmeterol xinafoate AEROSOL, METERED | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Prasco Laboratories(065969375) |
| REGISTRANT - GlaxoSmithKline LLC(167380711) |
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
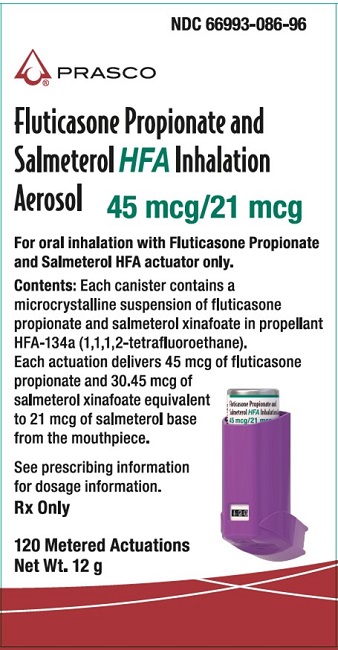
NDC 66993-086-96
Fluticasone Propionate and Salmeterol HFA Inhalation Aerosol
45 mcg/21 mcg
PRASCO
For oral inhalation with Fluticasone Propionate and Salmeterol HFA actuator only.
Contents: Each canister contains a microcrystalline suspension of fluticasone propionate and salmeterol xinafoate in propellant HFA-134a (1,1,1,2-tetrafluoroethane).
Each actuation delivers 45 mcg of fluticasone propionate and 30.45 mcg of salmeterol xinafoate equivalent to 21 mcg of salmeterol base from the mouthpiece.
See prescribing information for dosage information.
RX Only
120 Metered Actuations
Net Wt. 12 g
- 62000000083185 Rev. 10/22
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 66993-087-96
Fluticasone Propionate and Salmeterol HFA Inhalation Aerosol
115 mcg/21 mcg
PRASCO
For oral inhalation with Fluticasone Propionate and Salmeterol HFA actuator only.
Contents: Each canister contains a microcrystalline suspension of fluticasone propionate and salmeterol xinafoate in propellant HFA-134a (1,1,1,2-tetrafluoroethane).
Each actuation delivers 115 mcg of fluticasone propionate and 30.45 mcg of salmeterol xinafoate equivalent to 21 mcg of salmeterol base from the mouthpiece.
See prescribing information for dosage information.
RX Only
120 Metered Actuations
Net Wt. 12 g
- 62000000083187 Rev. 10/22

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 66993-088-96
Fluticasone Propionate and Salmeterol HFA Inhalation Aerosol
230 mcg/21 mcg
PRASCO
For oral inhalation with Fluticasone Propionate and Salmeterol HFA actuator only.
Contents: Each canister contains a microcrystalline suspension of fluticasone propionate and salmeterol xinafoate in propellant HFA-134a (1,1,1,2-tetrafluoroethane).
Each actuation delivers 230 mcg of fluticasone propionate and 30.45 mcg of salmeterol xinafoate equivalent to 21 mcg of salmeterol base from the mouthpiece.
See prescribing information for dosage information.
RX Only
120 Metered Actuations
Net Wt. 12 g
- 6200000083189 Rev. 10/22










