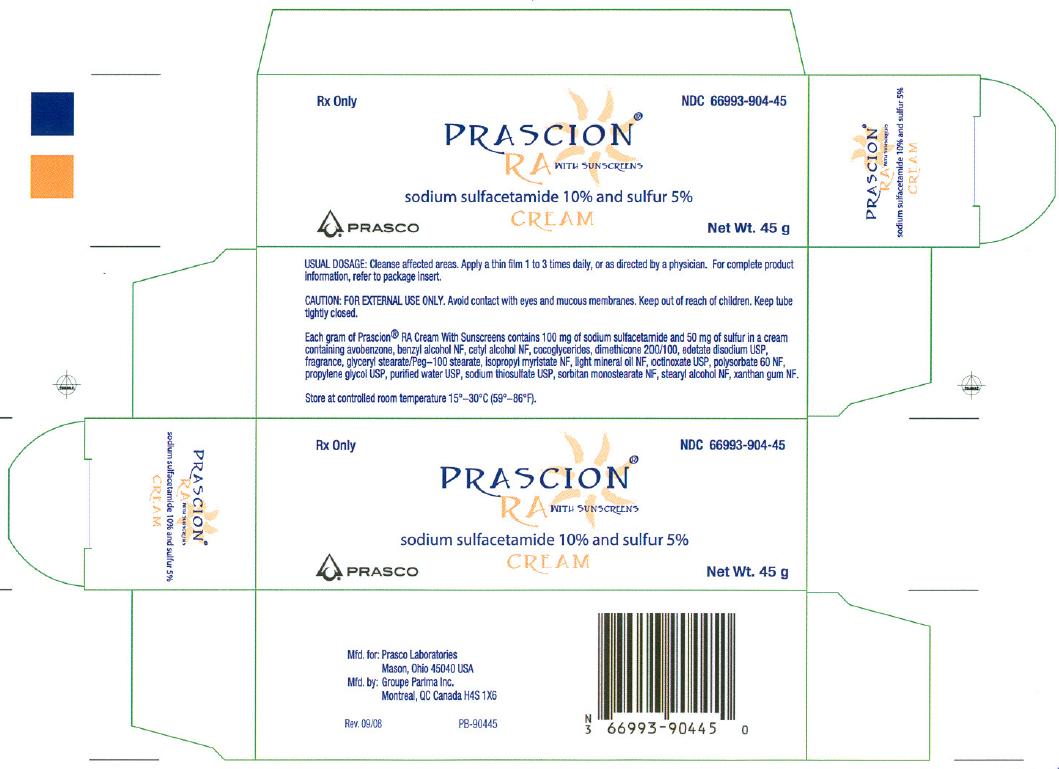
NDC Code(s) : 66993-904-45
Packager : Prasco Laboratories
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Prascion Sodium Sulfacetamide and Sulfur CREAM | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Manufactured for: Prasco Laboratories
Mason, OH 45040 USA
Manufactured by: Groupe Parima, Inc.
Montreal, QC H4S 1X6 CANADA