NDC Code(s) : 67386-130-51, 67386-130-91
Packager : Lundbeck Pharmaceuticals LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Vyeptieptinezumab-jjmr INJECTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Lundbeck Pharmaceuticals LLC(009582068) |
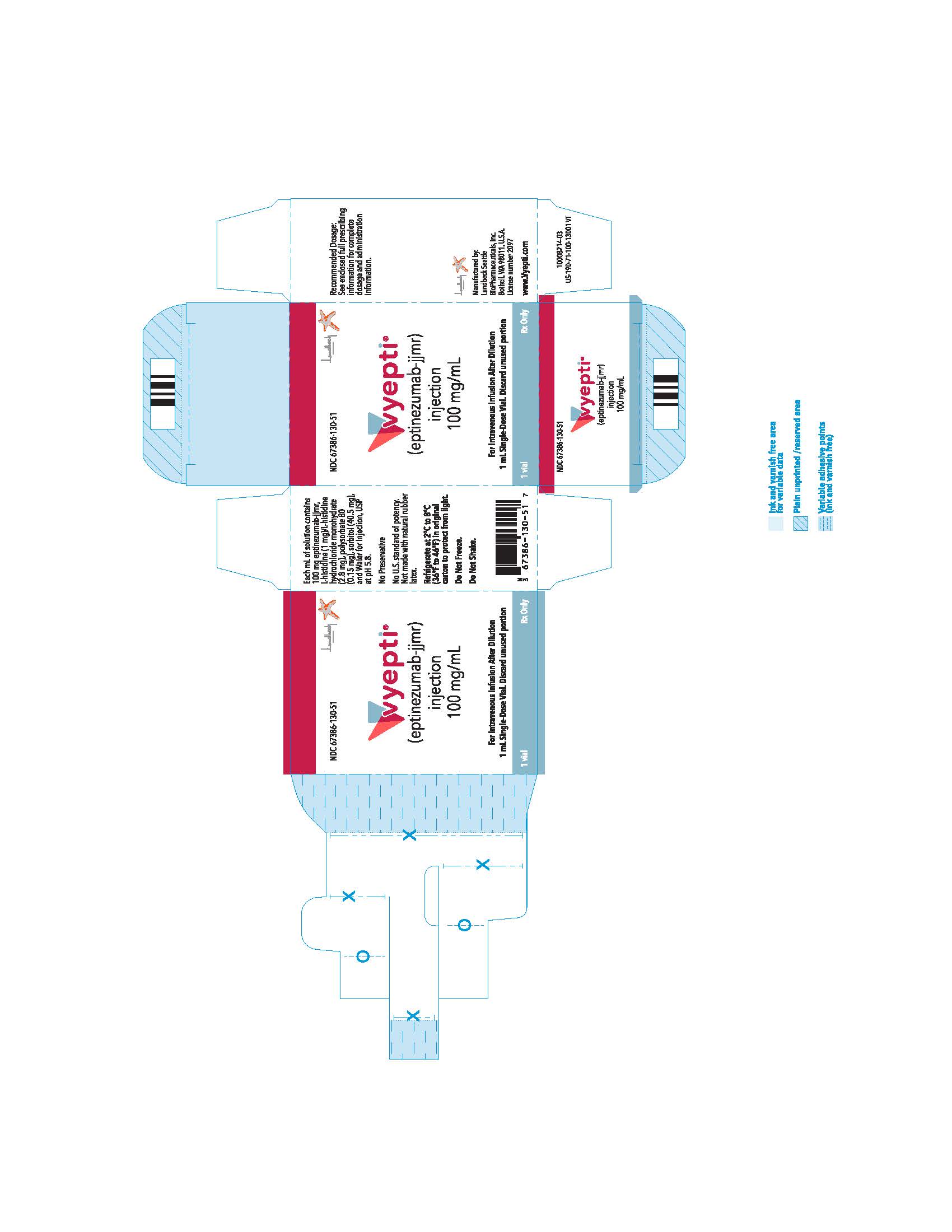
PRINCIPAL DISPLAY PANEL
NDC 67386-130-51
VYEPTITM (vye ep' tee)
(eptinezumab-jjmr)
injection, for intravenous use
100 mg/mL
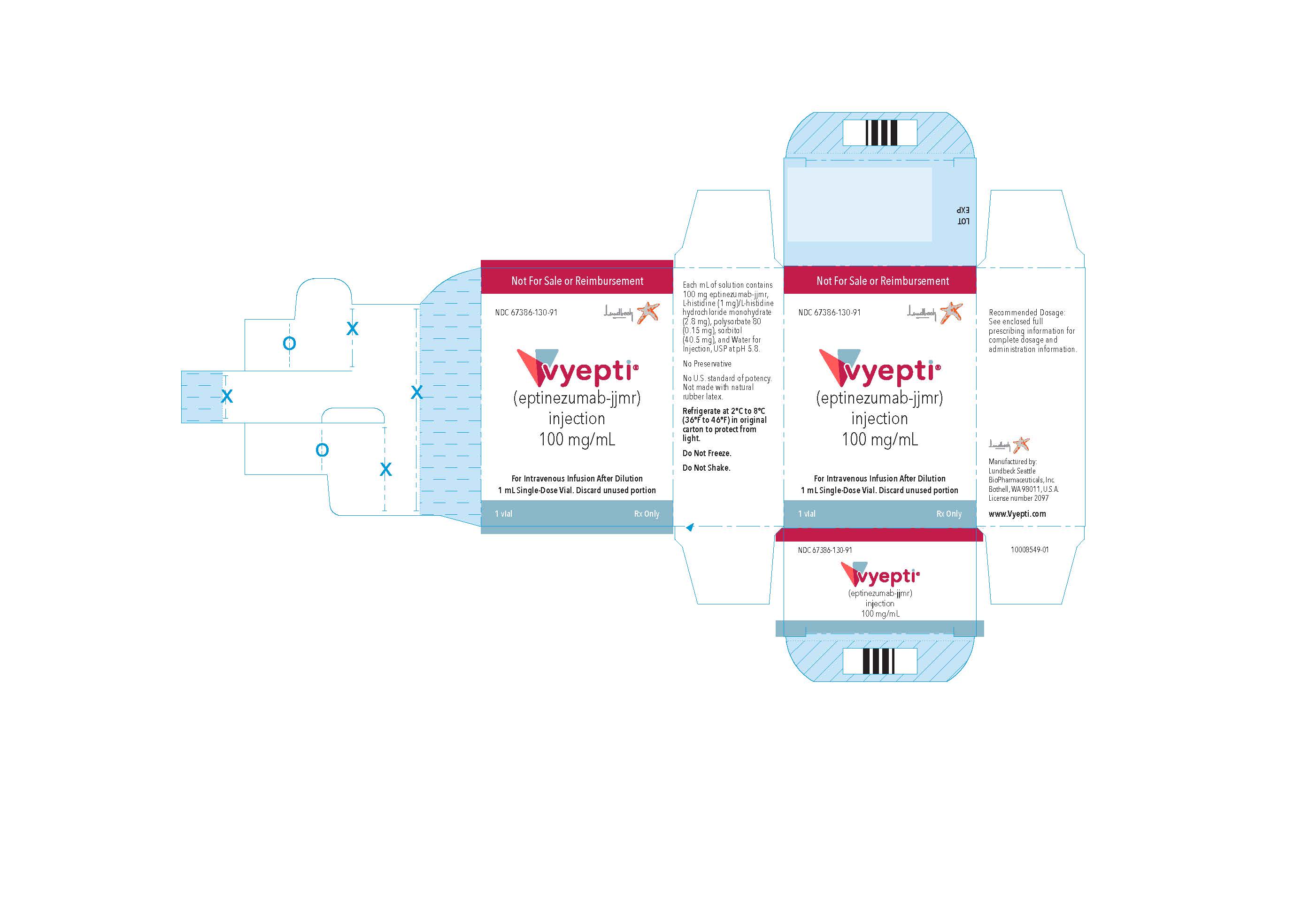
PRINCIPAL DISPLAY PANEL
NDC 67386-130-91
VYEPTITM (vye ep' tee)
(eptinezumab-jjmr)
injection, for intravenous use
100 mg/mL
Professional Sample
Professional Sample









