NDC Code(s) : 67457-225-00, 67457-225-10, 67457-222-00, 67457-222-20
Packager : Mylan Institutional LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Vancomycin HydrochlorideVancomycin Hydrochloride INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Vancomycin HydrochlorideVancomycin Hydrochloride INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 67457-225-10
Vancomycin HCl
for Injection, USP
500 mg
Equivalent to
500 mg vancomycin
For Intravenous Use Only
AFTER RECONSTITUTION MUST BE FURTHER DILUTED
BEFORE USE. SEE ACCOMPANYING PRESCRIBING
INFORMATION.
Rx only 10 x 500 mg Sterile Vials
IMPORTANT: READ INSERT FOR
PRECAUTIONS AND DIRECTIONS BEFORE
USE.
Preservative Free. Sterile. Lyophilized.
Each vial contains: Vancomycin hydrochloride
equivalent to 500 mg vancomycin.
Usual Adult Dosage: 2 g daily in divided
doses. See accompanying prescribing
information.
Dilute contents of the vial with 10 mL Sterile
Water for injection.
Prior to reconstitution: Store dry powder at
20° to 25°C (68° to 77°F). [See USP Controlled
Room Temperature.]
After reconstitution: This vial may be stored in
a refrigerator for 96 hours without significant
loss of potency.
Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103 U.S.A.
Manufactured by MN Pharmaceuticals
Made in Turkey MI:225:10C:R2

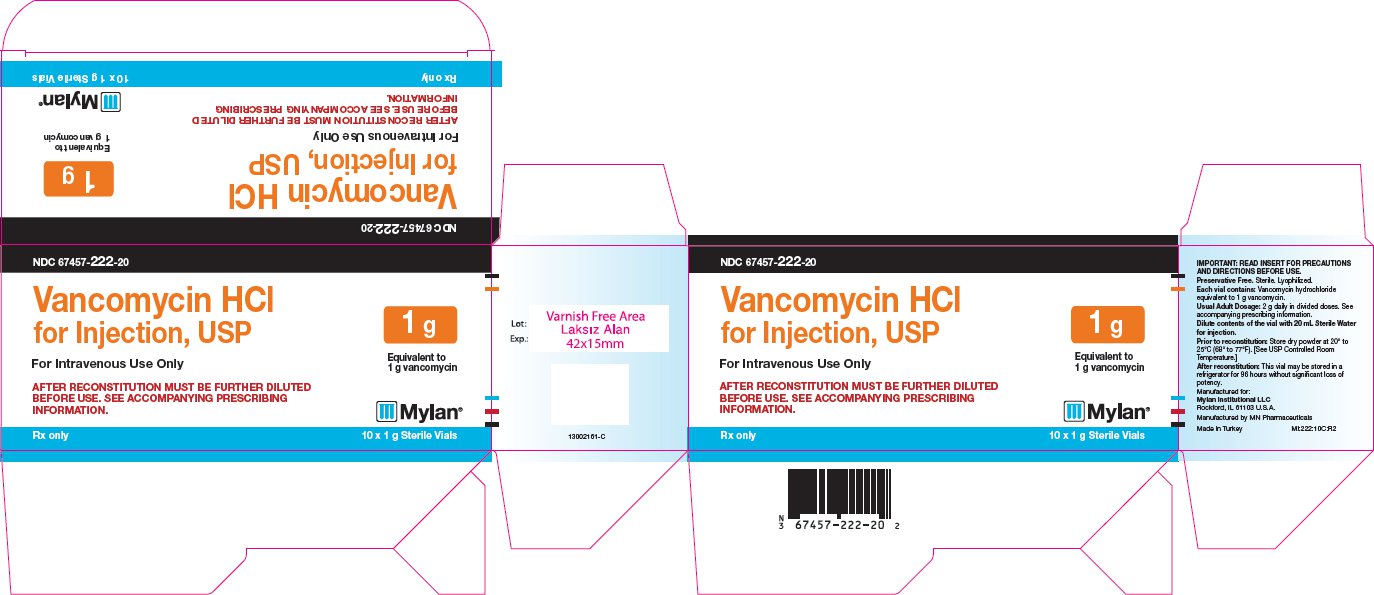
PRINCIPAL DISPLAY PANEL
NDC 67457-222-20
Vancomycin HCl
for Injection, USP
1 g
Equivalent to
1 g vancomycin
For Intravenous Use Only
AFTER RECONSTITUTION MUST BE FURTHER DILUTED
BEFORE USE. SEE ACCOMPANYING PRESCRIBING
INFORMATION.
Rx only 10 x 1 g Sterile Vials
IMPORTANT: READ INSERT FOR
PRECAUTIONS AND DIRECTIONS BEFORE
USE.
Preservative Free. Sterile. Lyophilized.
Each vial contains: Vancomycin hydrochloride
equivalent to 1 g vancomycin.
Usual Adult Dosage: 2 g daily in divided doses. See
accompanying prescribing information.
Dilute contents of the vial with 20 mL Sterile
Water for injection.
Prior to reconstitution: Store dry powder at 20° to
25°C (68° to 77°F). [See USP Controlled Room
Temperature.]
After reconstitution: This vial may be stored in a
refrigerator for 96 hours without significant loss of
potency.
Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103 U.S.A.
Manufactured by MN Pharmaceuticals
Made in Turkey MI:222:10C:R2