NDC Code(s) : 67457-423-00, 67457-423-12, 67457-422-00, 67457-422-54, 67457-421-00, 67457-421-30, 67457-420-00, 67457-420-10
Packager : Mylan Institutional LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Dexamethasone Sodium PhosphateDexamethasone Sodium Phosphate INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Dexamethasone Sodium PhosphateDexamethasone Sodium Phosphate INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Dexamethasone Sodium PhosphateDexamethasone Sodium Phosphate INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Dexamethasone Sodium PhosphateDexamethasone Sodium Phosphate INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Mylan Institutional LLC(790384502) |
PRINCIPAL DISPLAY PANEL
NDC 67457-420-10
Dexamethasone
Sodium Phosphate
Injection, USP
100 mg/10 mL (10 mg/mL*)
Sterile
For IM or IV use only
Rx only
10 x 10 mL Multi-Dose Vials
*Each mL contains:
Active: Dexamethasone Sodium Phosphate 11 mg
(eq. to Dexamethasone Phosphate 10 mg)
Preservatives: Methylparaben 1.5 mg;
Propylparaben 0.2 mg
Inactives: Edetate Disodium 0.11 mg; Sodium
Citrate 10 mg; Citric Acid and/or Sodium Hydroxide
to adjust pH (7.0 to 8.5); and Water for Injection q.s.
to 1 mL.
Usual Dosage: See package insert.
Storage: Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
Protect from light. Sensitive to heat.
Do not autoclave. Protect from freezing.
Manufactured for:
Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.
Made in India
Code No.: TN/DRUGS/TN00003234
Mylan.com

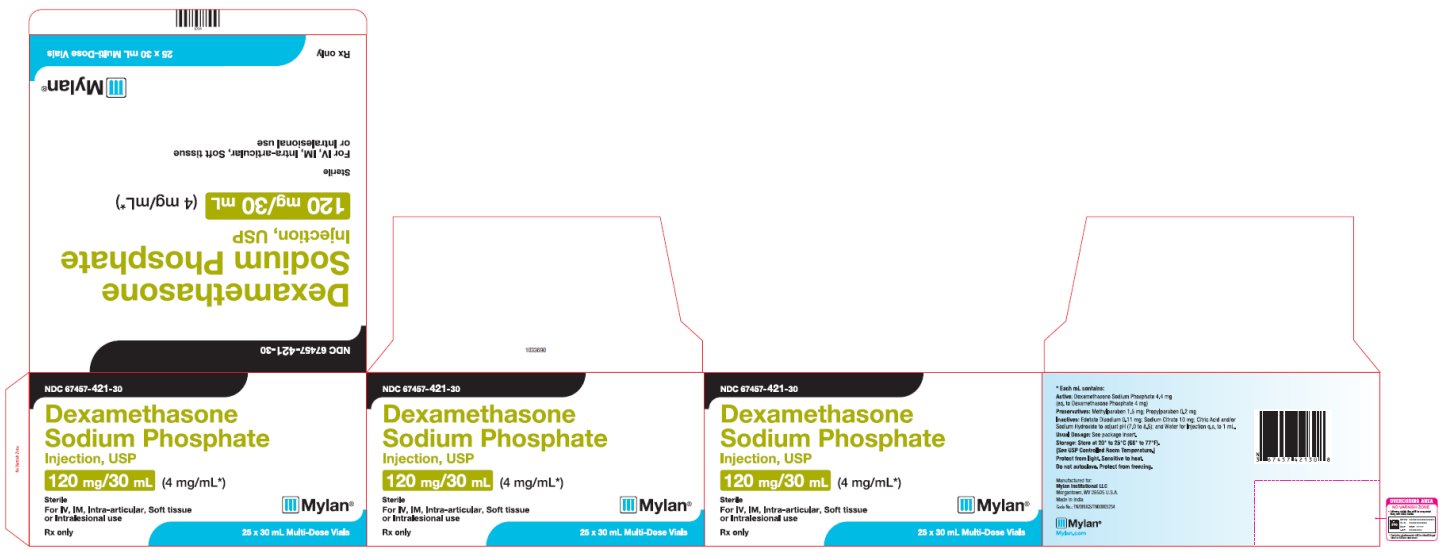
PRINCIPAL DISPLAY PANEL
NDC 67457-421-30
Dexamethasone
Sodium Phosphate
Injection, USP
120 mg/30 mL (4 mg/mL*)
Sterile
For IV, IM, Intra-articular, Soft tissue
or Intralesional use
Rx only
25 x 30 mL Multi-Dose Vials
*Each mL contains:
Active: Dexamethasone Sodium Phosphate 4.4 mg
(eq. to Dexamethasone Phosphate 4 mg)
Preservatives: Methylparaben 1.5 mg; Propylparaben 0.2 mg
Inactives: Edetate Disodium 0.11 mg; Sodium Citrate 10 mg; Citric Acid and/or
Sodium Hydroxide to adjust pH (7.0 to 8.5); and Water for Injection q.s. to 1 mL.
Usual Dosage: See package insert.
Storage: Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
Protect from light. Sensitive to heat.
Do not autoclave. Protect from freezing.
Manufactured for:
Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.
Made in India
Code No.: TN/DRUGS/TN00003234
Mylan.com
PRINCIPAL DISPLAY PANEL
NDC 67457-422-54
Dexamethasone
Sodium Phosphate
Injection, USP
20 mg/5 mL (4 mg/mL*)
Sterile
For IV, IM, Intra-articular, Soft tissue
or Intralesional use
Rx only
25 x 5 mL Multi-Dose Vials
*Each mL contains:
Active: Dexamethasone Sodium Phosphate 4.4 mg
(eq. to Dexamethasone Phosphate 4 mg)
Preservatives: Methylparaben 1.5 mg; Propylparaben 0.2 mg
Inactives: Edetate Disodium 0.11 mg; Sodium Citrate 10 mg; Citric Acid and/or
Sodium Hydroxide to adjust pH (7.0 to 8.5); and Water for Injection q.s. to 1 mL.
Usual Dosage: See package insert.
Storage: Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
Protect from light. Sensitive to heat.
Do not autoclave. Protect from freezing.
Manufactured for:
Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.
Made in India
Code No.: TN/DRUGS/TN00003234
Mylan.com

PRINCIPAL DISPLAY PANEL
NDC 67457-423-12
Dexamethasone
Sodium Phosphate
Injection, USP
4 mg/mL*
Sterile
For IV, IM, Intra-articular
Soft tissue or Intralesional use
Rx only
25 x 1 mL Single-Dose Vials
*Each mL contains:
Active: Dexamethasone Sodium Phosphate 4.4 mg
(eq. to Dexamethasone Phosphate 4 mg)
Preservatives: Methylparaben 1.5 mg; Propylparaben 0.2 mg
Inactives: Edetate Disodium 0.11 mg; Sodium Citrate 10 mg;
Citric Acid and/or Sodium Hydroxide to adjust pH (7.0 to 8.5);
and Water for Injection q.s. to 1 mL.
Usual Dosage: See package insert.
Storage: Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
Protect from light. Sensitive to heat.
Do not autoclave. Protect from freezing.
Manufactured for:
Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.
Made in India
Code No.: TN/DRUGS/TN00003234
Mylan.com










