NDC Code(s) : 67457-571-00, 67457-571-10, 67457-572-00, 67457-572-20
Packager : Mylan Institutional LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| VECURONIUM BROMIDEVECURONIUM BROMIDE INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| VECURONIUM BROMIDEVECURONIUM BROMIDE INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
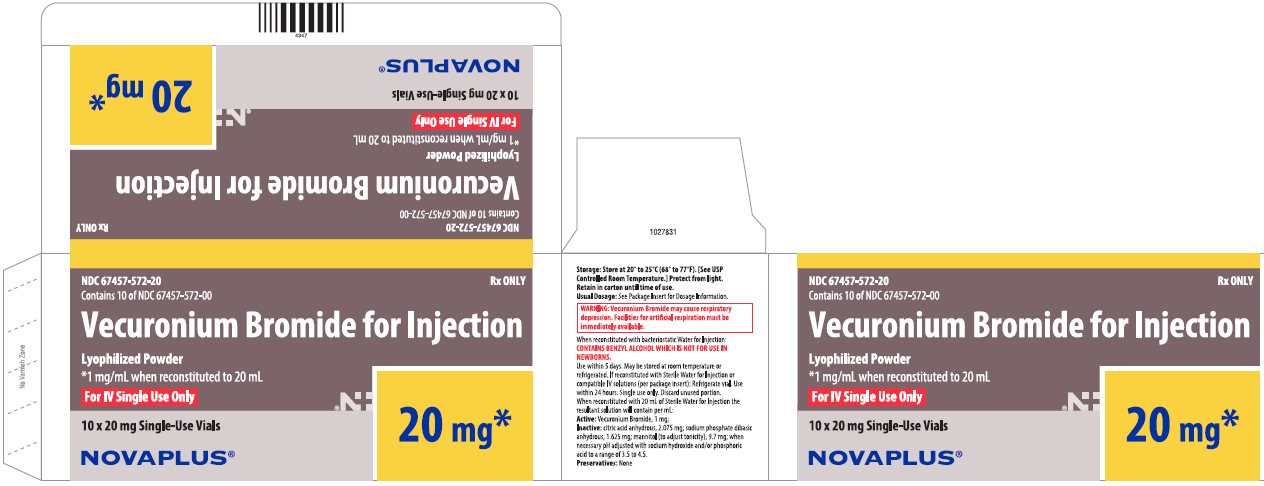
PRINCIPAL DISPLAY PANEL
NDC 67457-571-10
Rx ONLY
Contains 10 of NDC 67457-571-00
Vecuronium Bromide for Injection
Lyophilized Powder
*1 mg/mL when reconstituted to 10 mL
For IV Single Use Only
10 x 10 mg Single-Use Vials
10 mg*
NOVAPLUS
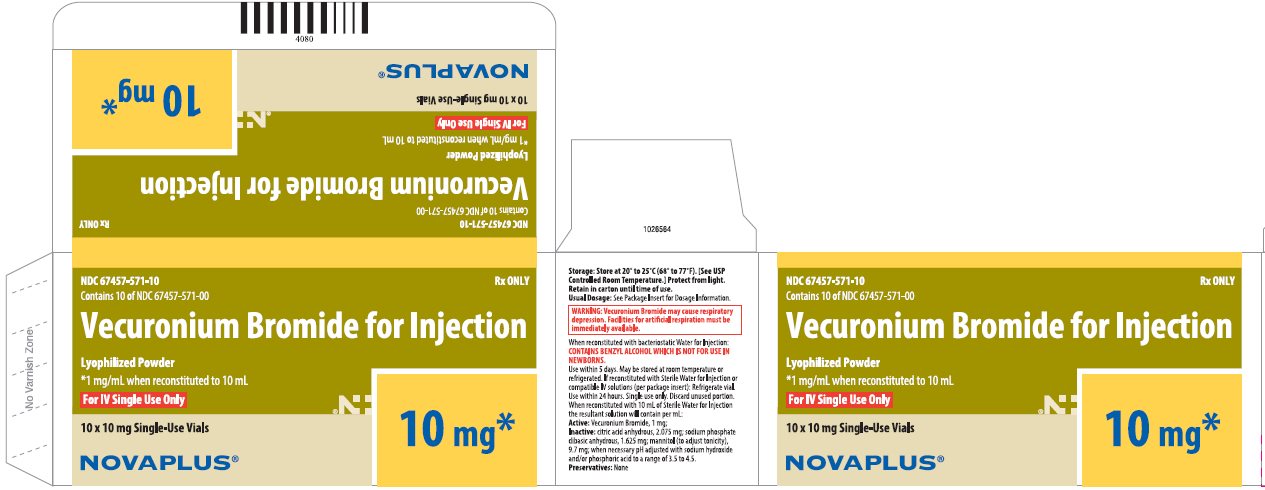
PRINCIPAL DISPLAY PANEL
NDC 67457-572-20
Rx ONLY
Contains 10 of NDC 67457-572-00
Vecuronium Bromide for Injection
Lyophilized Powder
*1 mg/mL when reconstituted to 20 mL
For IV Single Use Only
10 x 20 mg Single-Use Vials
20 mg*
NOVAPLUS