NDC Code(s) : 67457-580-00, 67457-580-02
Packager : Mylan Institutional LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Dexmedetomidine Hydrochloridedexmedetomidine hydrochloride INJECTION, SOLUTION, CONCENTRATE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
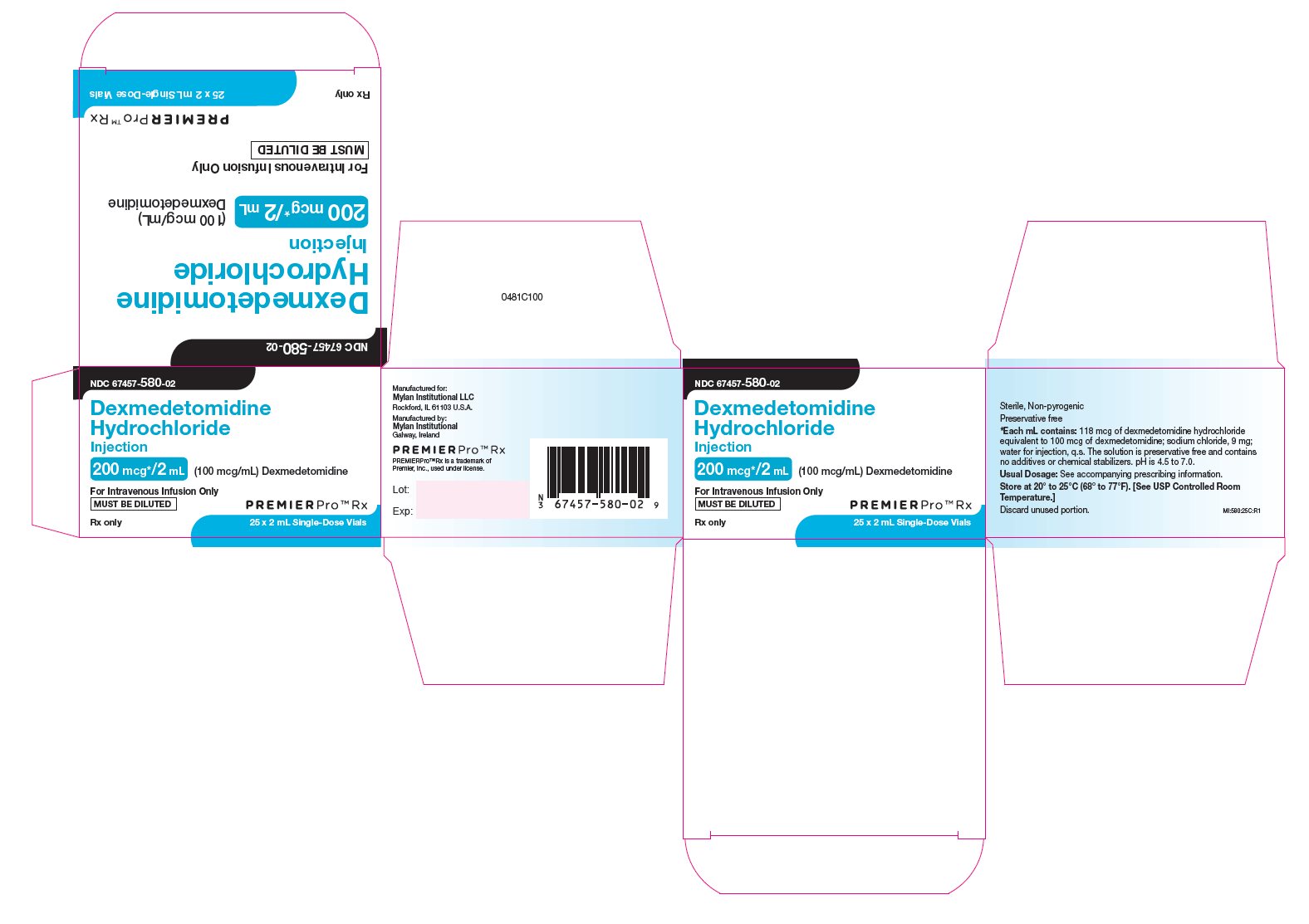
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 200 mcg*/2 mL
NDC 67457-580-02
Dexmedetomidine
Hydrochloride
Injection
200 mcg*/2 mL
(100 mcg/mL) Dexmedetomidine
For Intravenous Infusion Only
MUST BE DILUTED
Rx only 25 x 2 mL Single-Dose Vials
Sterile, Nonpyrogenic
Preservative free
*Each mL contains: 118 mcg of dexmedetomidine hydrochloride
equivalent to 100 mcg of dexmedetomidine; sodium chloride, 9 mg;
water for injection, q.s. The solution is preservative free and contains
no additives or chemical stabilizers. pH is 4.5 to 7.0.
Usual Dosage: See accompanying prescribing information.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room
Temperature.]
Discard unused portion.
MI:580:25C:R1
Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103 U.S.A.
Manufactured by:
Mylan Institutional
Galway, Ireland
PREMIERPro™Rx is a trademark of
Premier, Inc., used under license.