NDC Code(s) : 67457-887-00, 67457-887-99, 67457-887-01
Packager : Mylan Institutional LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Medroxyprogesterone AcetateMedroxyprogesterone Acetate INJECTION, SUSPENSION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Mylan Institutional LLC(790384502) |
PRINCIPAL DISPLAY PANEL
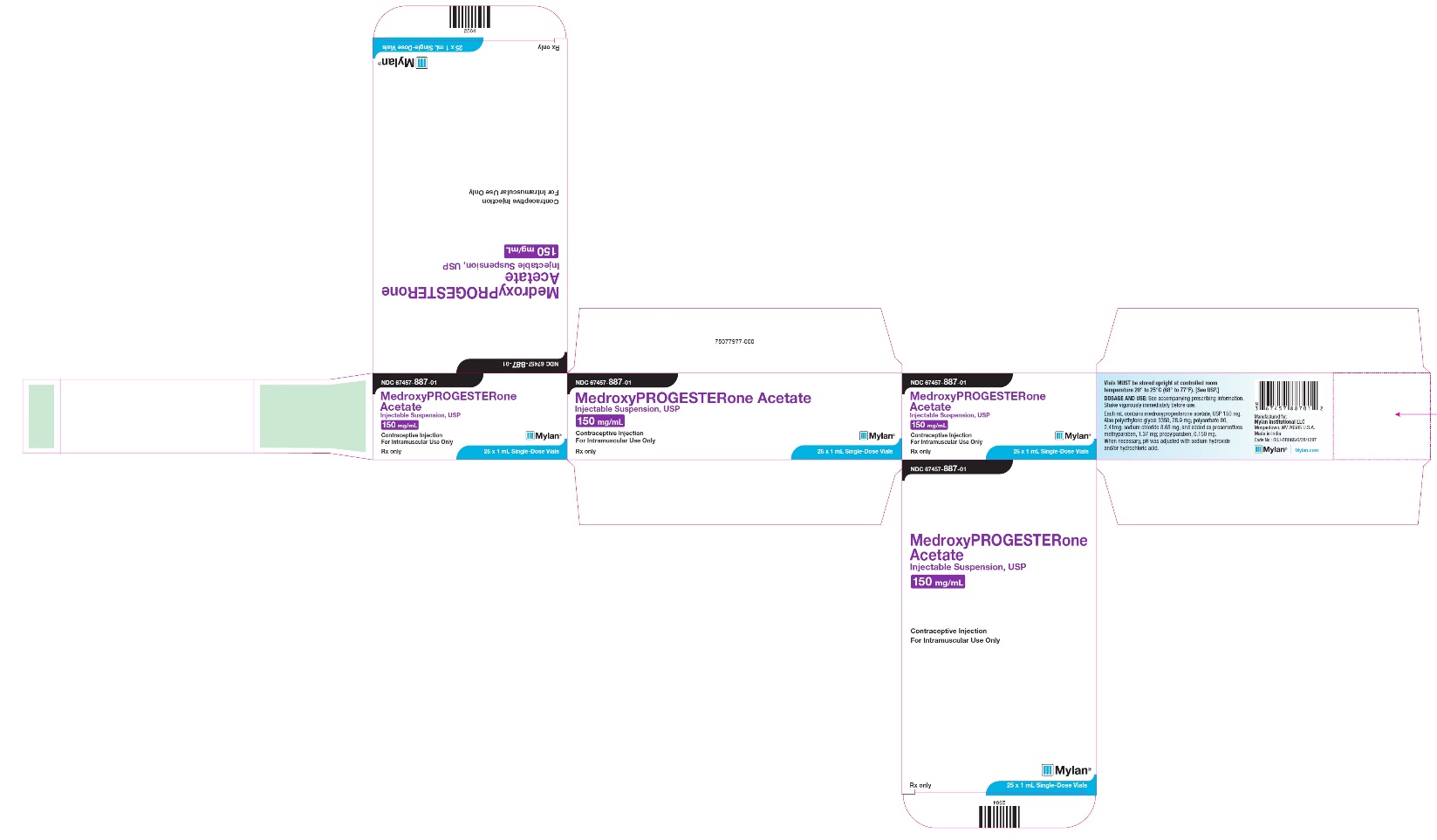
NDC 67457-887-01
MedroxyPROGESTERone Acetate Injectable Suspension, USP
150 mg/mL
Contraceptive Injection
For Intramuscular Use Only
Rx only
Mylan
25 x 1 mL Single-Dose Vials
PRINCIPAL DISPLAY PANEL
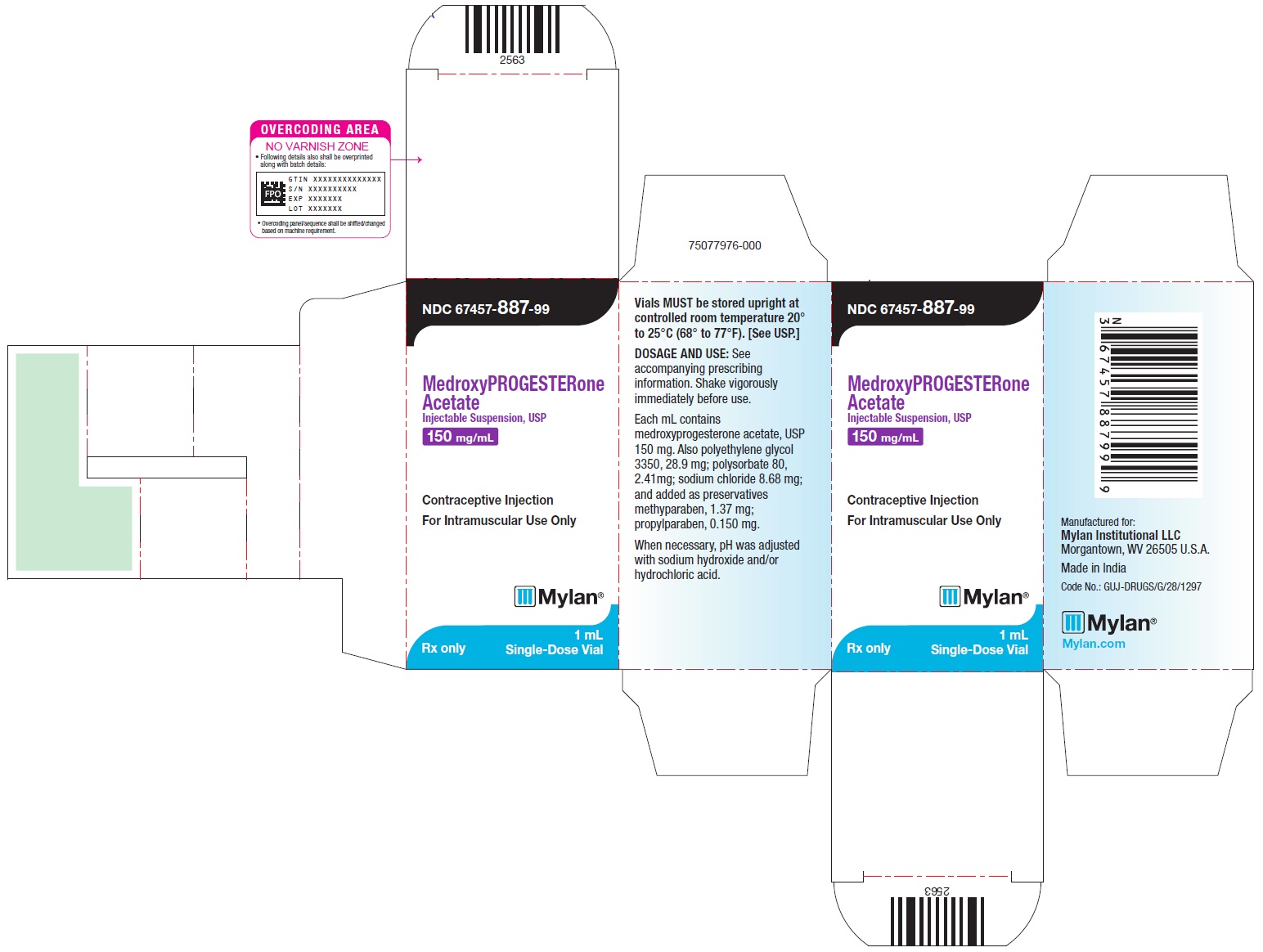
NDC 67457-887-99
MedroxyPROGESTERone Acetate Injectable Suspension, USP
150 mg/mL
Contraceptive Injection
For Intramuscular Use Only
Rx only
Mylan
1 mL Single-Dose Vial
PRINCIPAL DISPLAY PANEL
NDC 67457-887-00
MedroxyPROGESTERone Acetate Injectable Suspension, USP
150 mg/mL
Contraceptive Injection
For Intramuscular Use Only
Rx only
Mylan
1 mL Single-Dose Vial










