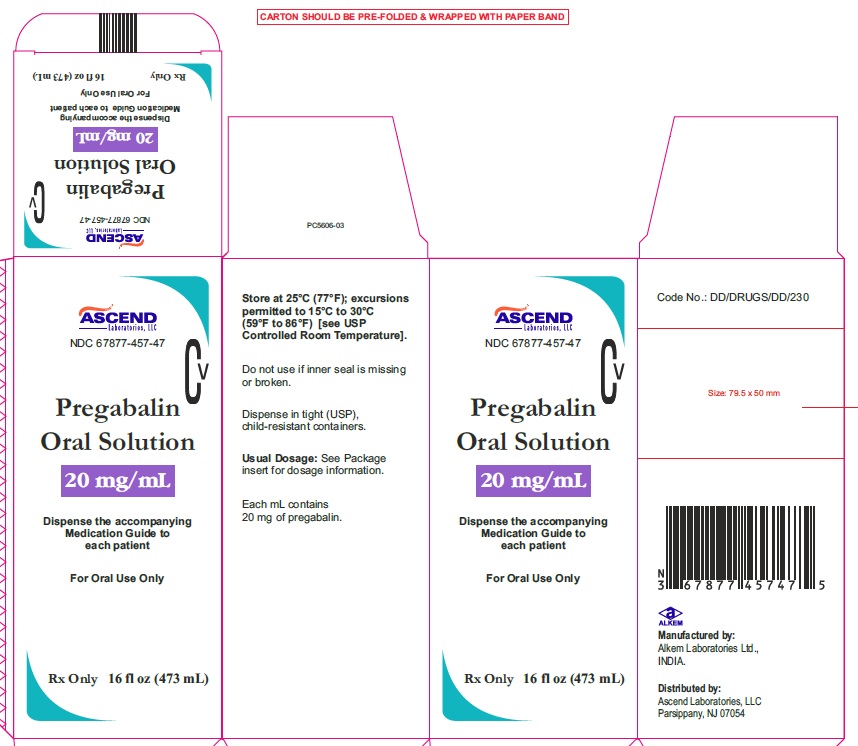
NDC Code(s) : 67877-457-47
Packager : Ascend Laboratories, LLC
Category : Human Prescription Drug Label
DEA Schedule : CV
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Pregabalin Pregabalin SOLUTION | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| LABELER - Ascend Laboratories, LLC(141250469) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Alkem Laboratories Limited | 915628612 | MANUFACTURE(67877-457) | |
PRINCIPAL DISPLAY PANEL
NDC 67877-457-47
Pregabalin Oral Solution
20 mg/ml
Rx Only
16 fl oz (473 ml)

NDC 67877-457-47
Pregabalin Oral Solution
20 mg/ml
Rx Only
16 fl oz (473 ml) (Carton label)