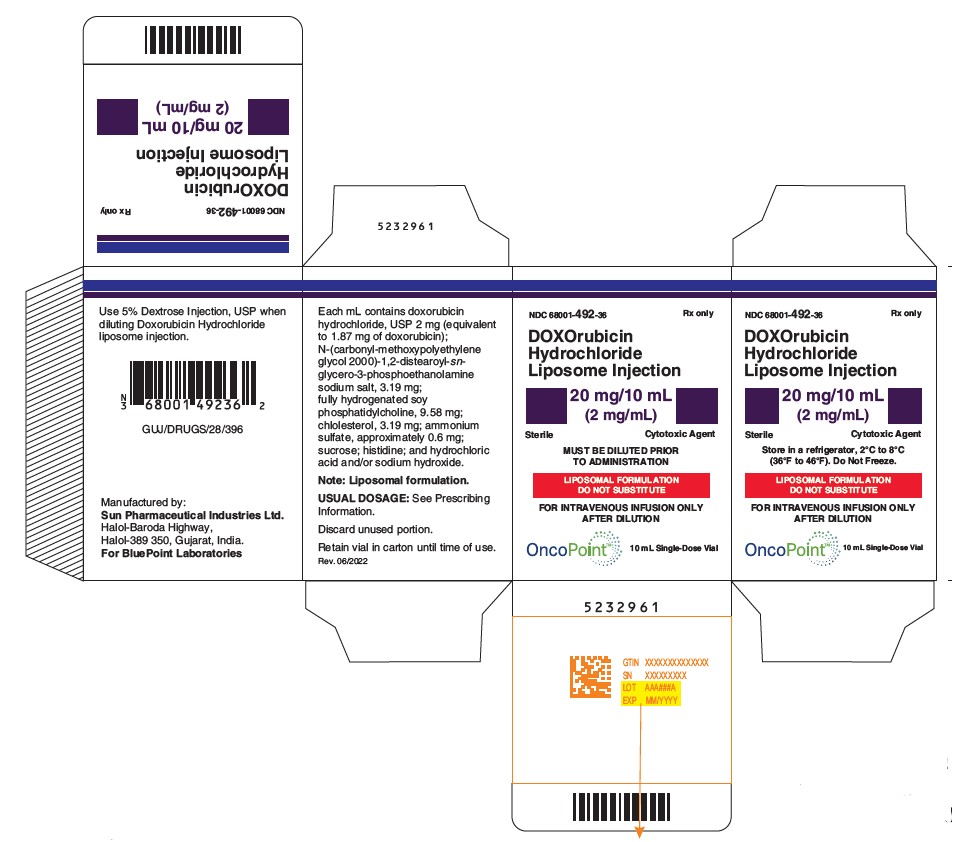
NDC Code(s) : 68001-492-36, 68001-493-26
Packager : BluePoint Laboratories
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Doxorubicin HydrochlorideDoxorubicin Hydrochloride INJECTABLE, LIPOSOMAL | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Doxorubicin HydrochlorideDoxorubicin Hydrochloride INJECTABLE, LIPOSOMAL | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - BluePoint Laboratories(985523874) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Sun Pharmaceutical Industries Limited | 725959238 | analysis(68001-492, 68001-493), manufacture(68001-492, 68001-493) | |
PRINCIPAL DISPLAY PANEL
NDC 68001-492-36
DOXOrubicin Hydrochloride Liposome Injection
20 mg/10 mL (2 mg/mL)
Cytotoxic Agent
Must be diluted
LIPOSOMAL FORMULATION
DO NOT SUBSTITUTE
FOR INTRAVENOUS INFUSION ONLY AFTER DILUTION
Store in a refrigerator, 2°C to 8°C (36°F to 46°F).
Do Not Freeze.

PRINCIPAL DISPLAY PANEL
NDC 68001-492-36
DOXOrubicin Hydrochloride Liposome Injection
20 mg/10 mL (2 mg/mL)
Sterile
Cytotoxic Agent
MUST BE DILUTED PRIOR TO ADMINISTRATION
LIPOSOMAL FORMULATION
DO NOT SUBSTITUTE
FOR INTRAVENOUS INFUSION ONLY AFTER DILUTION
Rx only
10 mL Single-dose Vial
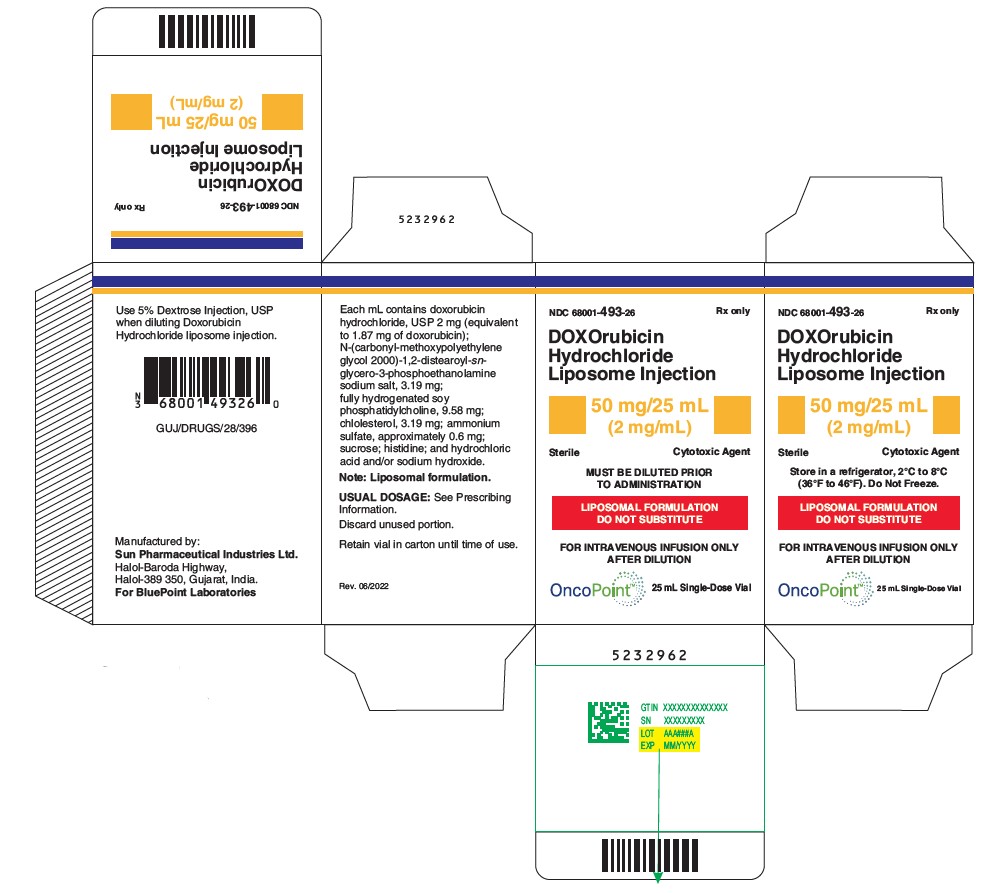
PRINCIPAL DISPLAY PANEL
NDC 68001-493-26
DOXOrubicin Hydrochloride Liposome Injection
50 mg/25 mL (2 mg/mL)
Cytotoxic Agent
Must be diluted
LIPOSOMAL FORMULATION
DO NOT SUBSTITUTE
FOR INTRAVENOUS INFUSION ONLY AFTER DILUTION
Store in a refrigerator, 2°C to 8°C (36°F to 46°F).
Do Not Freeze.

PRINCIPAL DISPLAY PANEL
NDC 68001-493-26
DOXOrubicin Hydrochloride Liposome Injection
50 mg/25 mL (2 mg/mL)
Sterile
Cytotoxic Agent
MUST BE DILUTED PRIOR TO ADMINISTRATION
LIPOSOMAL FORMULATION
DO NOT SUBSTITUTE
FOR INTRAVENOUS INFUSION ONLY AFTER DILUTION
Rx only
25 mL Single-dose Vial