NDC Code(s) : 68001-512-06, 68001-513-06, 68001-514-06, 68001-515-06
Packager : Bluepoint Laboratories
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| QUETIAPINE FUMARATEQUETIAPINE FUMARATE TABLET, EXTENDED RELEASE | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| QUETIAPINE FUMARATEQUETIAPINE FUMARATE TABLET, EXTENDED RELEASE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| QUETIAPINE FUMARATEQUETIAPINE FUMARATE TABLET, EXTENDED RELEASE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| QUETIAPINE FUMARATEQUETIAPINE FUMARATE TABLET, EXTENDED RELEASE | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| LABELER - Bluepoint Laboratories(985523874) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Intas Pharmaceuticals Limited | 725927649 | manufacture(68001-512, 68001-513, 68001-514, 68001-515), analysis(68001-512, 68001-513, 68001-514, 68001-515), pack(68001-512, 68001-513, 68001-514, 68001-515) | |
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 68001-512-06
Quetiapine Extended-release Tablets USP150 mg*
Once Daily
Rx Only
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
60 Tablets

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
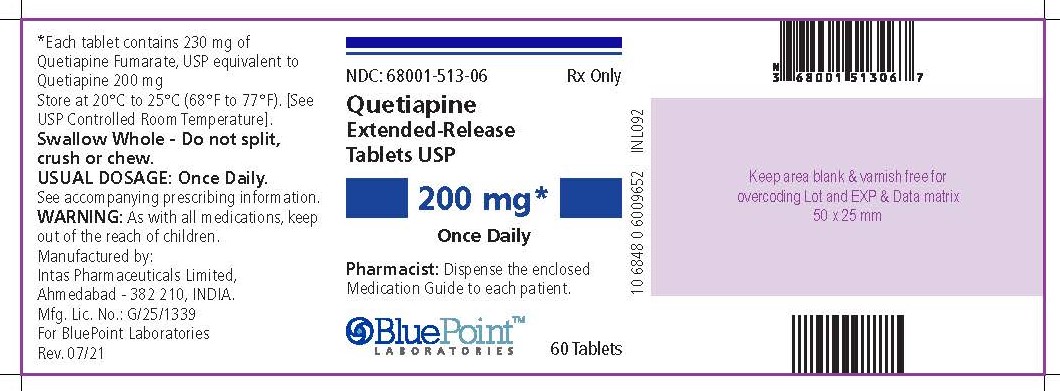
NDC 68001-513-06
Quetiapine Extended-release Tablets USP200 mg*
Once Daily
Rx Only
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
60 Tablets
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
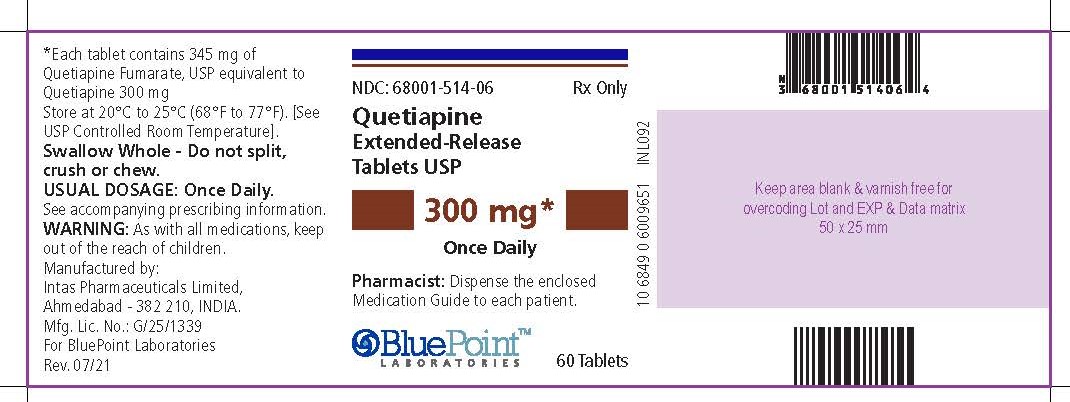
NDC 68001-514-06
Quetiapine Extended-release Tablets USP300 mg*
Once Daily
Rx Only
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
60 Tablets
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 68001-515-06
Quetiapine Extended-release Tablets USP400 mg*
Once Daily
Rx Only
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
60 Tablets










