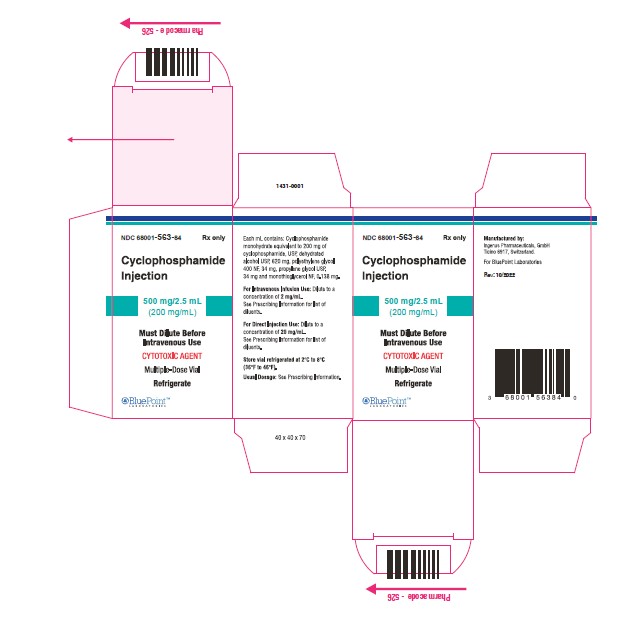
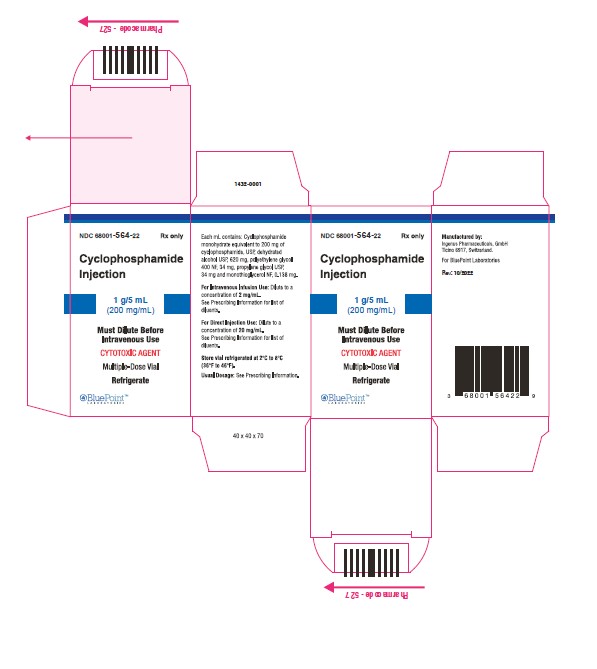
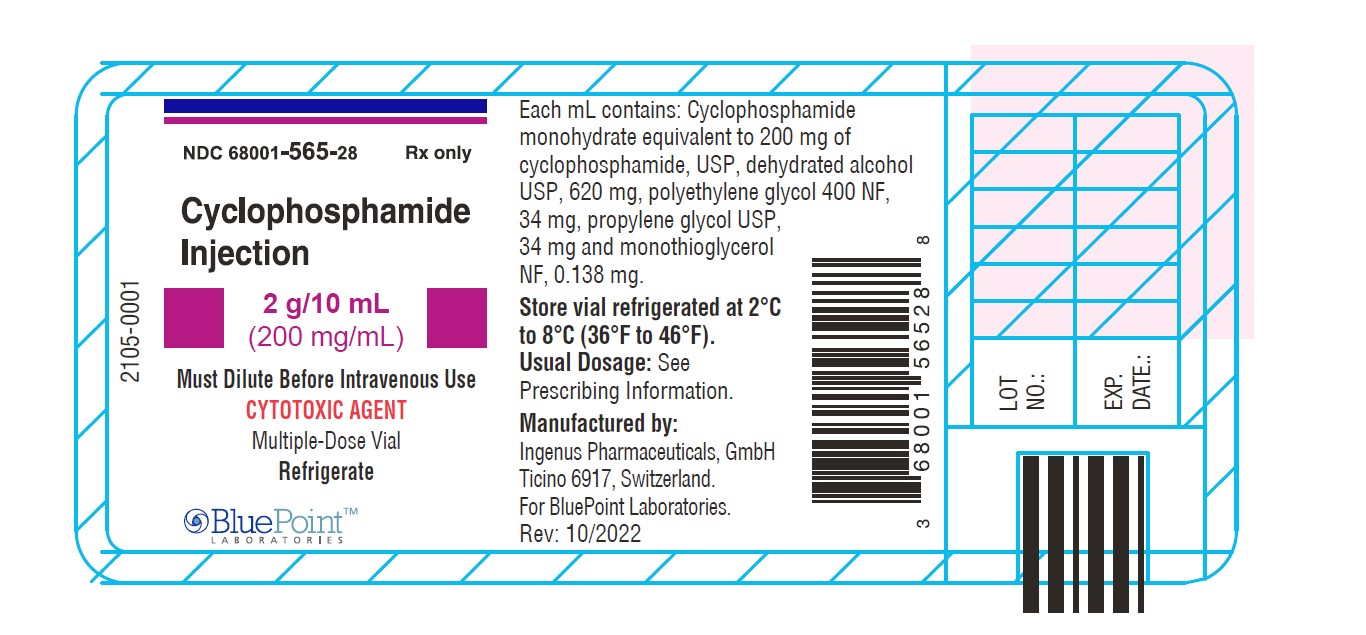
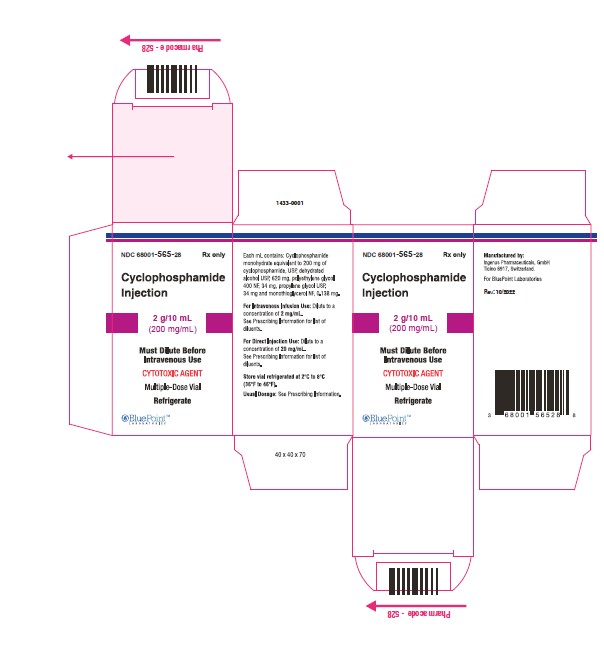
NDC Code(s) : 68001-563-84, 68001-564-22, 68001-565-28
Packager : BluePoint Laboratories
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CYCLOPHOSPHAMIDEcyclophosphamide INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CYCLOPHOSPHAMIDEcyclophosphamide INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CYCLOPHOSPHAMIDEcyclophosphamide INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - BluePoint Laboratories(985523874) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Ingenus Pharmaceuticals GmbH | 482730327 | analysis(68001-563, 68001-564, 68001-565), manufacture(68001-563, 68001-564, 68001-565), pack(68001-563, 68001-564, 68001-565), sterilize(68001-563, 68001-564, 68001-565) | |
PRINCIPAL DISPLAY PANEL