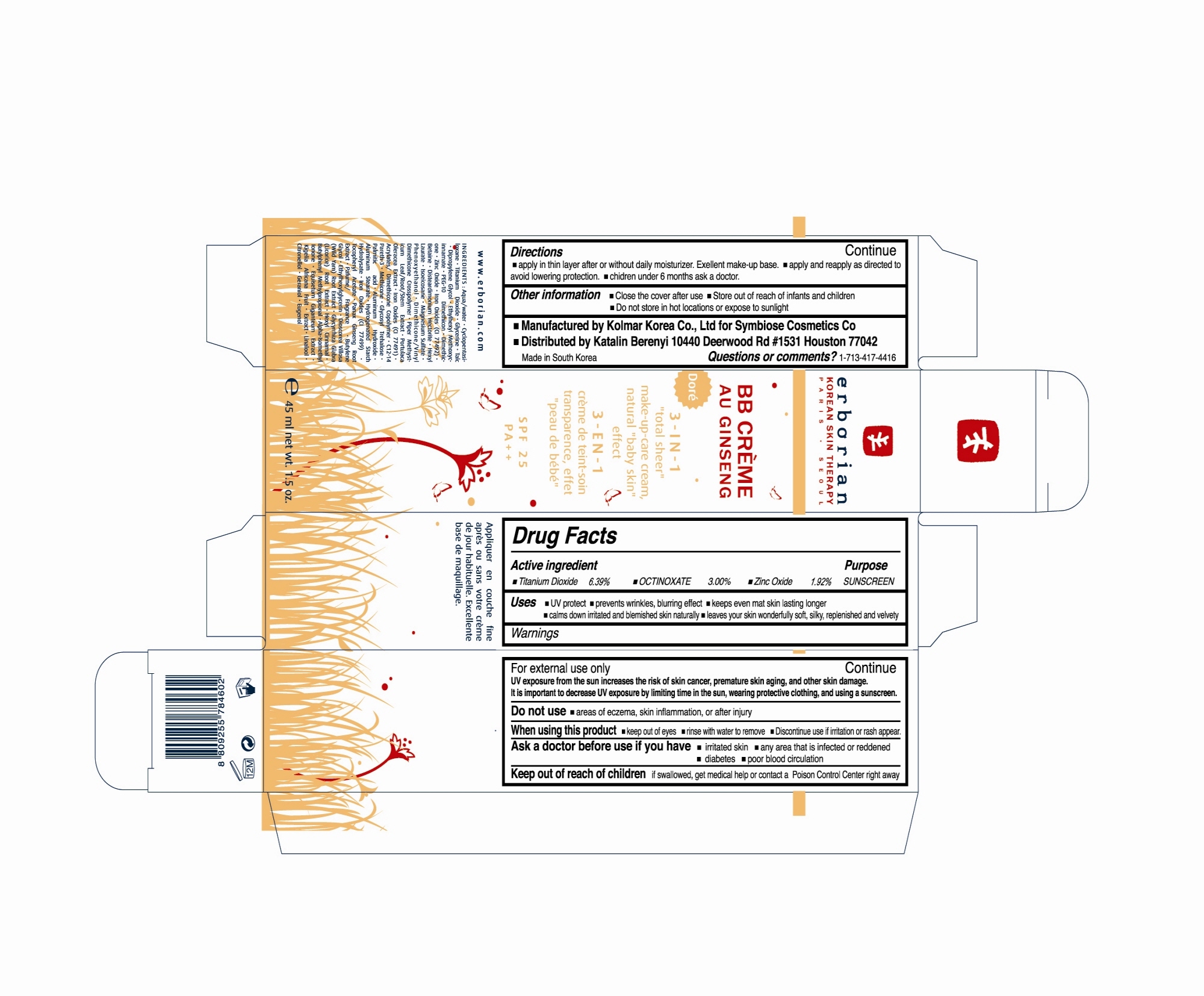
NDC Code(s) : 68021-1002-1, 68021-1002-2
Packager : Kolmar Korea Co Ltd
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Erborian BB Creme Au Ginseng Dore Titanium Dioxide CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
SYMBIOSE COSMETICS
Net WT. 1.5 OZ (45mL)