NDC Code(s) : 68083-295-01, 68083-296-01, 68083-439-01
Packager : Gland Pharma Limited
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Bimatoprost Bimatoprost SOLUTION/ DROPS | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Bimatoprost Bimatoprost SOLUTION/ DROPS | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Bimatoprost Bimatoprost SOLUTION/ DROPS | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Gland Pharma Limited(918601238) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| GLAND PHARMA LIMITED | 918601238 | ANALYSIS(68083-295, 68083-296, 68083-439), MANUFACTURE(68083-295, 68083-296, 68083-439), PACK(68083-295, 68083-296, 68083-439) | |
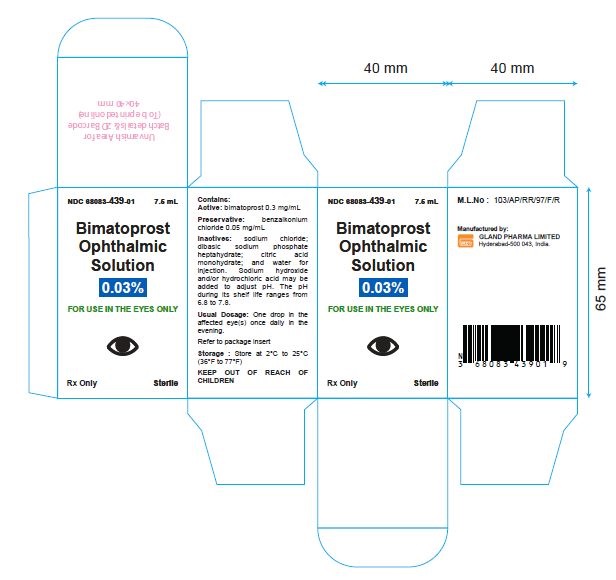
PRINCIPAL DISPLAY PANEL
Bottle Label - 2.5 mL:
NDC 68083-295-01 2.5 mL
Bimatoprost
Ophthalmic
Solution
0.03%
For Use in the Eyes Only
Rx Only sterile
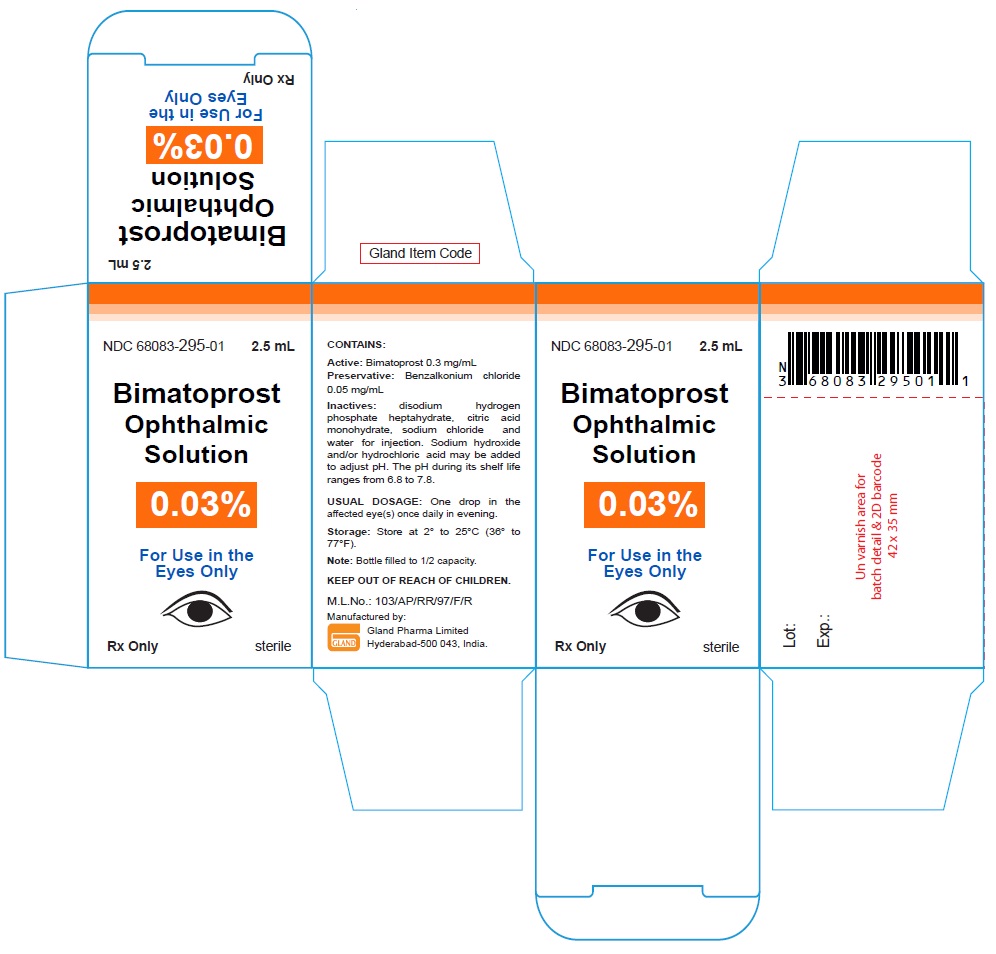
Carton Label - 2.5 mL:
NDC 68083-295-01 2.5 mL
Bimatoprost
Ophthalmic
Solution
0.03%
For Use in the
Eyes Only
Rx Only sterile
Bottle Label - 5 mL :
NDC 68083-296-01 5 mL
Bimatoprost
Ophthalmic
Solution
0.03%
For Use in the Eyes Only
Rx Only sterile

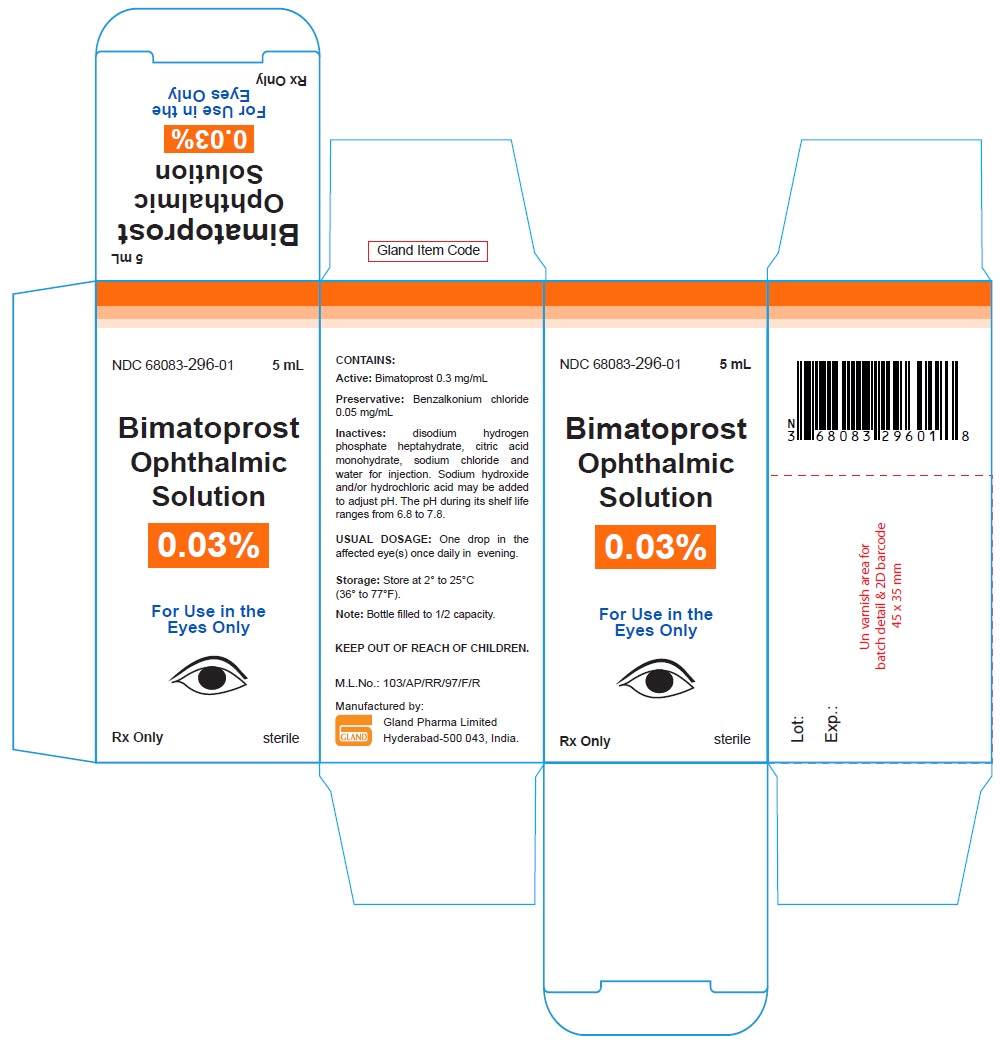
Carton Label - 5 mL:
NDC 68083-296-01 5 mL
Bimatoprost
Ophthalmic
Solution
0.03%
For Use in the
Eyes Only
Rx Only sterile
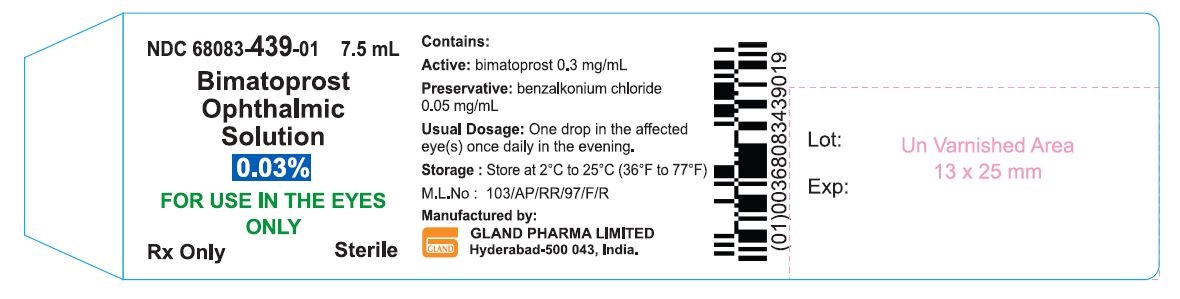
Bottle Label - 7.5 mL:
NDC 68083-439-01 7.5 mL
Bimatoprost
Ophthalmic
Solution
0.03%
For Use in the Eyes Only
Rx Only Sterile
Carton Label - 7.5 mL:
NDC 68083-439-01 7.5 mL
Bimatoprost
Ophthalmic
Solution
0.03%
For Use in the
Eyes Only
Rx Only sterile