NDC Code(s) : 68084-500-11, 68084-500-01, 68084-501-11, 68084-501-01, 68084-502-11, 68084-502-01, 68084-746-95, 68084-746-25
Packager : American Health Packaging
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| pravastatin sodiumpravastatin sodium TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| pravastatin sodiumpravastatin sodium TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| pravastatin sodiumpravastatin sodium TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| pravastatin sodiumpravastatin sodium TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL

NDC 68084-500-01
PRAVASTATIN
SODIUM
Tablets, USP
10 mg
100 Tablets (10 x 10)
Each Tablet Contains:
Pravastatin sodium, USP.............................. 10 mg
Usual Dosage: See package insert for full
prescribing information.
Store at 20° to 25°C (68° to 77°F); excursions
permitted to 15° to 30°C (59° to 86°F) [see USP
Controlled Room Temperature].
Protect from moisture and light.
Keep this and all drugs out of reach of children.
Rx Only
The drug product contained in this package is from
NDC # 68382-070, Zydus Pharmaceuticals USA Inc.
Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217
050001
Rev. 01/2015
PRINCIPAL DISPLAY PANEL
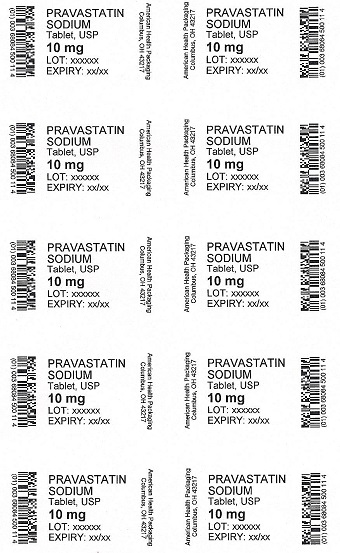
PRAVASTATIN
SODIUM
Tablet, USP
10 mg
PRINCIPAL DISPLAY PANEL

NDC 68084-501-01
PRAVASTATIN
SODIUM
Tablets, USP
20 mg
100 Tablets (10 x 10)
Each Tablet Contains:
Pravastatin sodium, USP.............................. 20 mg
Usual Dosage: See package insert for full
prescribing information.
Store at 20° to 25°C (68° to 77°F); excursions
permitted to 15° to 30°C (59° to 86°F) [see USP
Controlled Room Temperature].
Protect from moisture and light.
Keep this and all drugs out of reach of children.
Rx Only
The drug product contained in this package is from
NDC # 68382-071, Zydus Pharmaceuticals USA Inc.
Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217
050101
Rev. 01/2015
PRINCIPAL DISPLAY PANEL
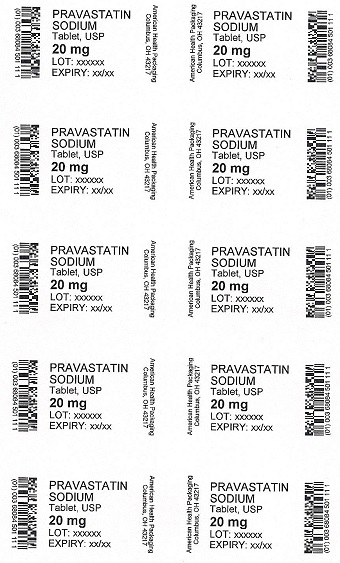
PRAVASTATIN
SODIUM
Tablet, USP
20 mg
PRINCIPAL DISPLAY PANEL

NDC 68084-502-01
PRAVASTATIN
SODIUM
Tablets, USP
40 mg
100 Tablets (10 x 10)
Each tablet contains:
Pravastatin sodium, USP................................40 mg
Usual Dosage: See package insert for complete
prescribing information.
Store at 20° to 25°C (68° to 77°F) [See USP
Controlled Room Temperature].
Protect from moisture and light.
Keep this and all drugs out of reach of children.
Rx only
The drug product contained in this package is
from NDC # 68382-072, Zydus Pharmaceuticals
(USA) Inc.
Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217
050201
Rev. 06/2013
PRINCIPAL DISPLAY PANEL
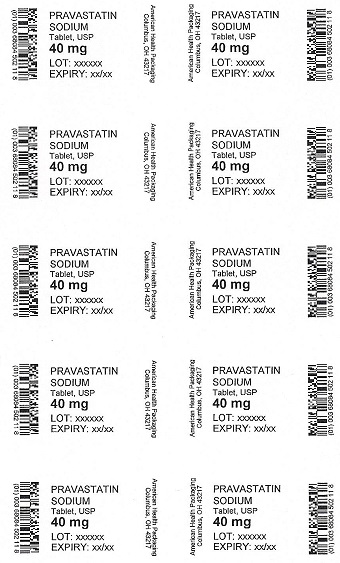
PRAVASTATIN
SODIUM
Tablet, USP
40 mg
PRINCIPAL DISPLAY PANEL

NDC 68084-746-25
PRAVASTATIN
SODIUM
Tablets, USP
80 mg
30 Tablets (5 x 6)
Each tablet contains:
Pravastatin sodium, USP........................80 mg
Usual Dosage: See package insert for
complete prescribing information.
Store at 20° to 25°C (68° to 77°F) [See USP
Controlled Room Temperature]. Protect
from light and moisture.
Keep this and all drugs out of reach of
children.
Rx Only
The drug product contained in this package
is from NDC # 68382-073, Zydus
Pharmaceuticals (USA) Inc.
Packaged and Distributed by:
American Health Packaging
Columbus, Ohio 43217
074625
Rev. 03/2014
PRINCIPAL DISPLAY PANEL

PRAVASTATIN
SODIUM
Tablet, USP
80 mg









