NDC Code(s) : 68093-7079-3
Packager : Wisconsin Pharmacal Company
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| VH Essentials Benzocaine, Benzalkonium Chloride CREAM | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
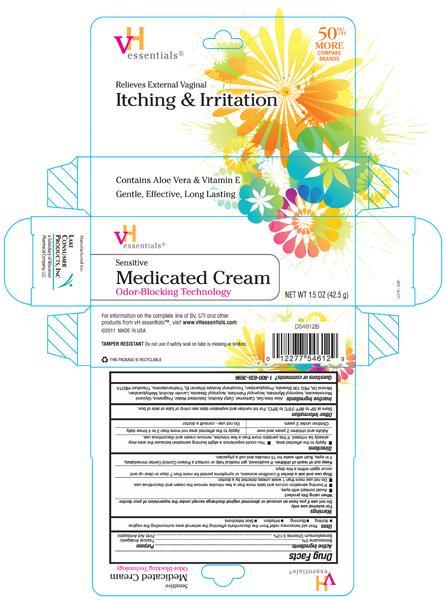
PRINCIPAL DISPLAY PANEL
Artwork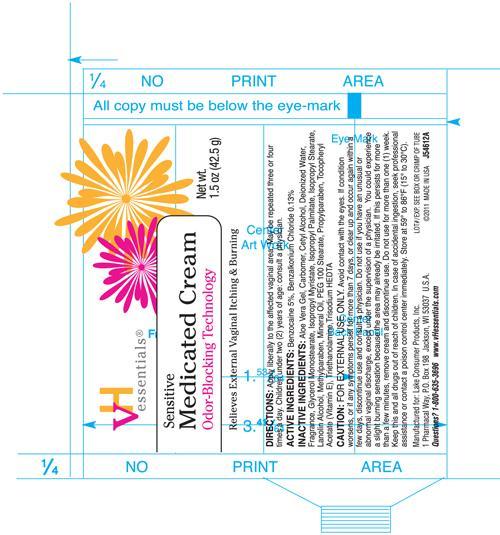
PRINCIPAL DISPLAY PANEL
Enter section text here