NDC Code(s) : 68180-260-01, 68180-260-02, 68180-260-06, 68180-261-01, 68180-261-02, 68180-261-06
Packager : Lupin Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Divalproex sodiumDivalproex sodium TABLET, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Divalproex sodiumDivalproex sodium TABLET, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| LABELER - Lupin Pharmaceuticals, Inc.(089153071) |
| REGISTRANT - LUPIN LIMITED(675923163) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LUPIN LIMITED | 650759348 | MANUFACTURE(68180-260, 68180-261), PACK(68180-260, 68180-261) | |
PRINCIPAL DISPLAY PANEL
NDC 68180-260-06
Bottle of 30 Tablets
Once-Daily Dosing
Divalproex Sodium Extended-release Tablets, USP
250 mg Valproic Acid Activity
Rx only
Dispense with Medication Guide provided separately to each patient.
 Image 5
Image 5
NDC 68180-261-06
Bottle of 30 Tablets
Once-Daily Dosing
Divalproex Sodium Extended-release Tablets, USP
500 mg Valproic Acid Activity
Rx only
Dispense with Medication Guide provided separately to each patient.
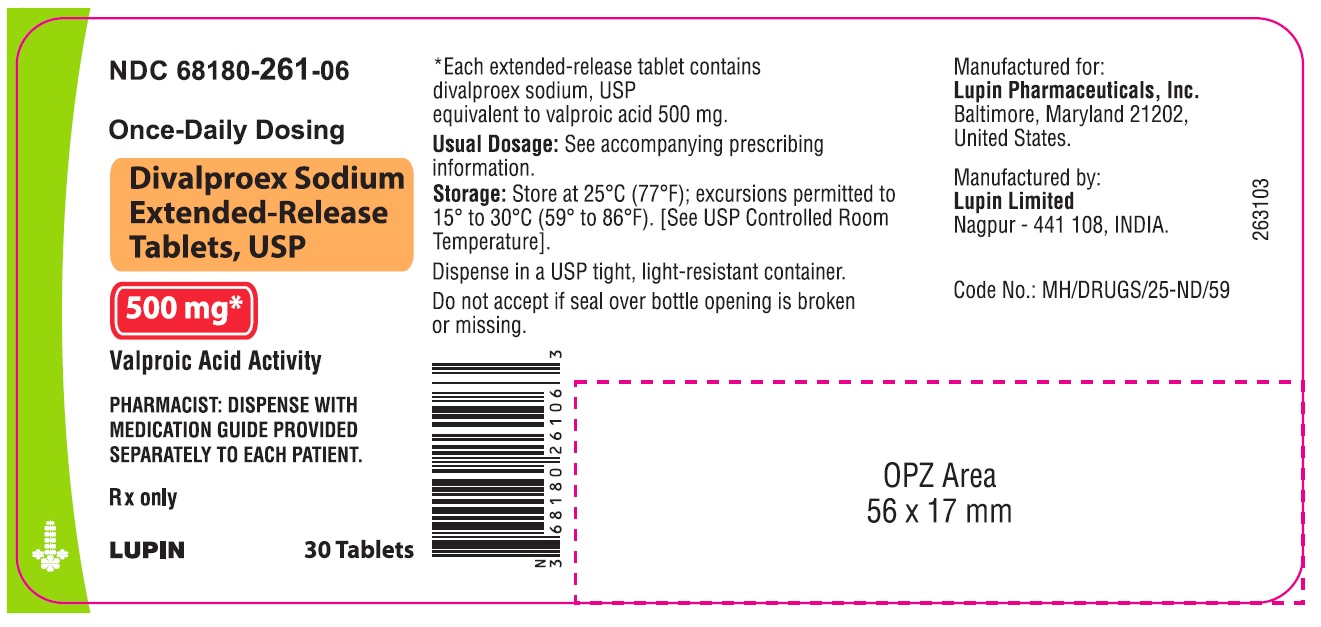 Image 6
Image 6









