NDC Code(s) : 68180-551-11, 68180-551-22
Packager : Lupin Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Clobetasol PropionateClobetasol Propionate SPRAY | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Lupin Pharmaceuticals, Inc.(089153071) |
| REGISTRANT - LUPIN LIMITED(675923163) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LUPIN LIMITED | 650595213 | MANUFACTURE(68180-551), PACK(68180-551) | |
PRINCIPAL DISPLAY PANEL
CLOBETASOL Propionate Spray, 0.05%
Rx Only
NDC 68180-551-22
For topical use only
4.25 FL OZ (125 mL) – Carton Label
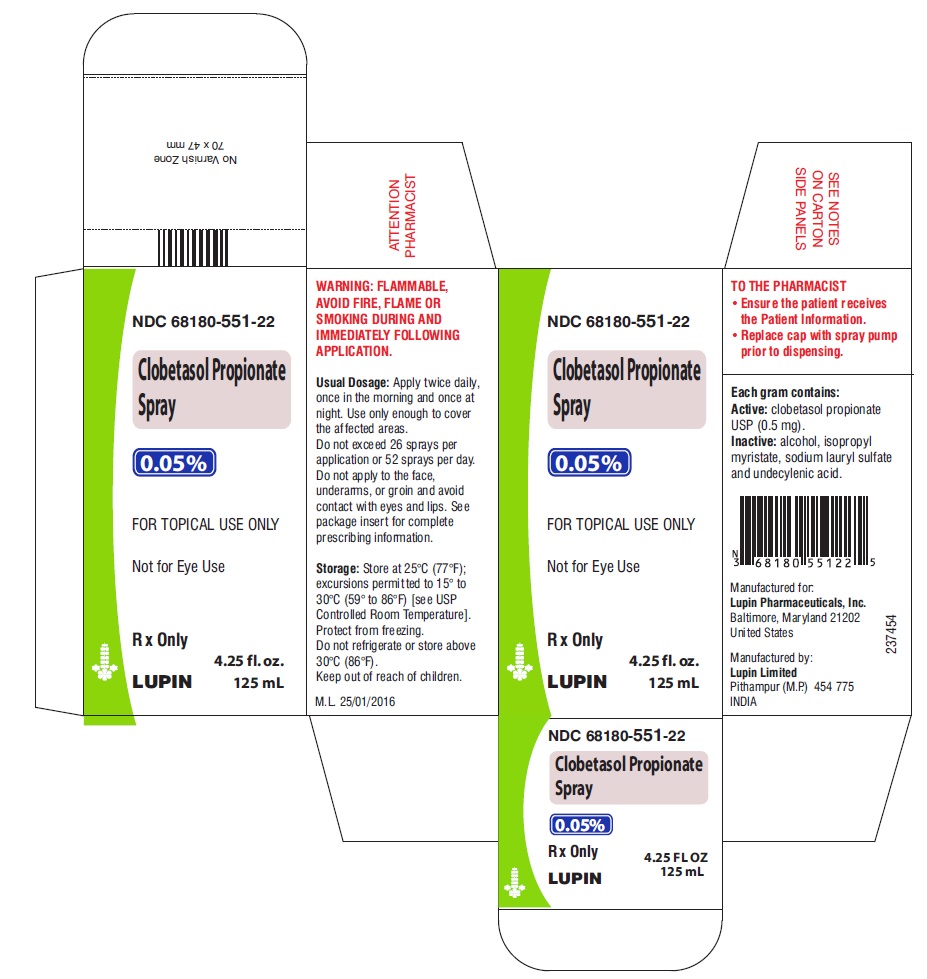
CLOBETASOL Propionate Spray, 0.05%
Rx Only
NDC 68180-551-22
For topical use only
4.25 FL OZ (125 mL) – Container Label










