NDC Code(s) : 68196-294-04
Packager : Sam's West Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| simply right minoxidilMinoxidil AEROSOL, FOAM | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
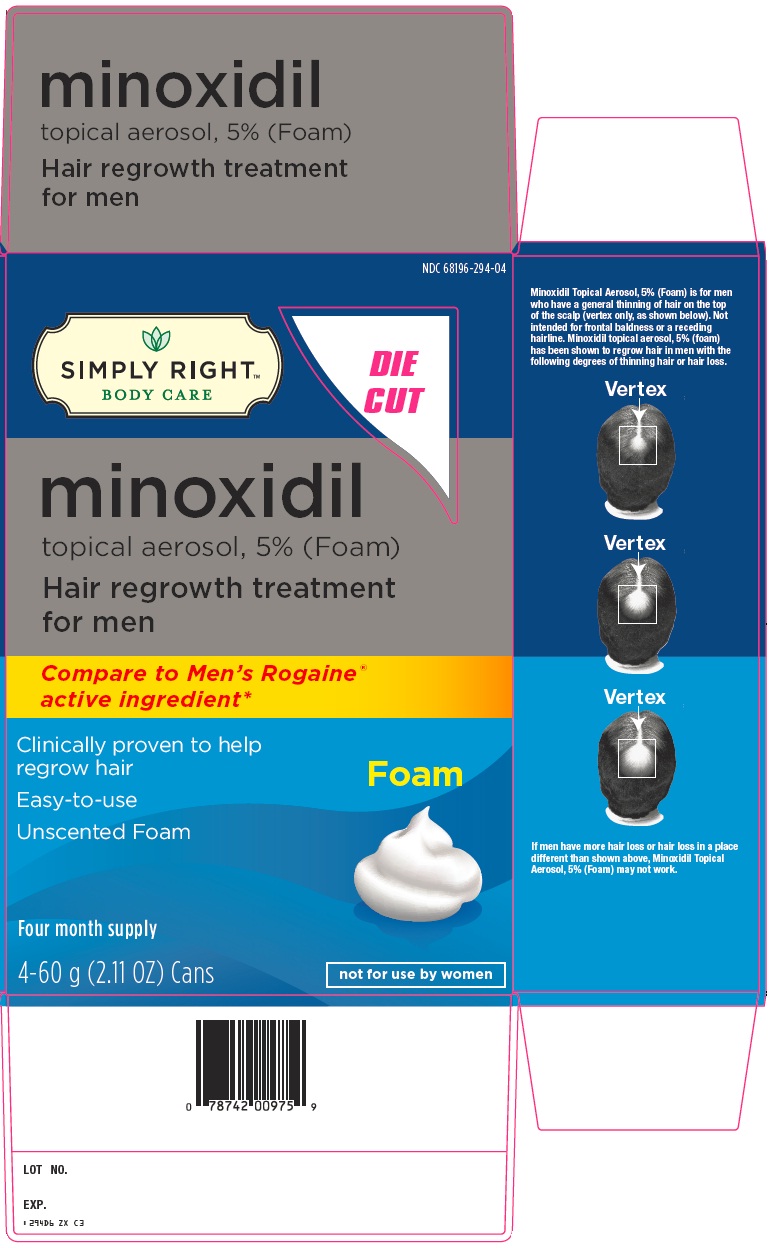
PRINCIPAL DISPLAY PANEL
minoxidil topical aerosol, 5% (Foam)
Hair regrowth treatment for men
Compare to Men’s Rogaine® active ingredient
Clinically proven to help regrow hair
Easy-to-use
Unscented Foam
Foam
Four month supply
4-60 g (2.11 OZ) Cans
not for use by women