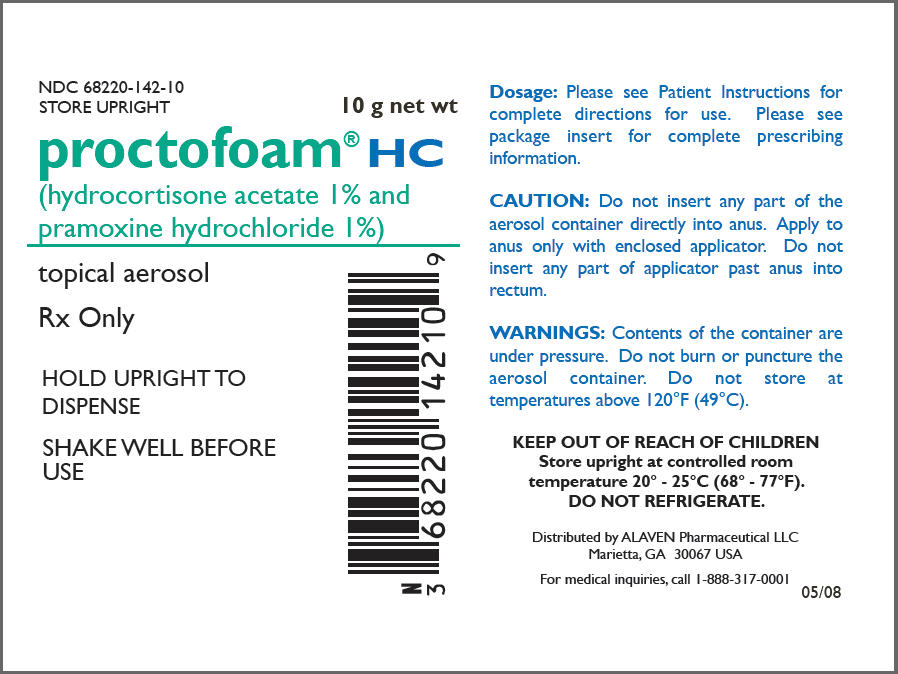
NDC Code(s) : 68220-142-10
Packager : Alaven Pharmaceutical LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Proctofoam hydrocortisone acetate and pramoxine hydrochloride AEROSOL, FOAM | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 68220-142-10
STORE UPRIGHT
10 g net wt
proctofoam® HC
(hydrocortisone acetate 1% and
pramoxine hydrochloride 1%)
topical aerosol
Rx Only
HOLD UPRIGHT TO
DISPENSE
SHAKE WELL BEFORE
USE