NDC Code(s) : 68327-030-01, 68327-031-01, 68327-032-01, 68327-033-01, 68327-034-01, 68327-035-01
Packager : Cover FX Skin Care, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Cover Fx Blemish Treatment Concealer N X-LightSALICYLIC ACID STICK | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Cover Fx Blemish Treatment Concealer N LightSALICYLIC ACID STICK | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Cover Fx Blemish Treatment Concealer N MediumSALICYLIC ACID STICK | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Cover Fx Blemish Treatment Concealer N Med-DeepSALICYLIC ACID STICK | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Cover Fx Blemish Treatment Concealer N DeepSALICYLIC ACID STICK | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Cover Fx Blemish Treatment Concealer N X-DeepSALICYLIC ACID STICK | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
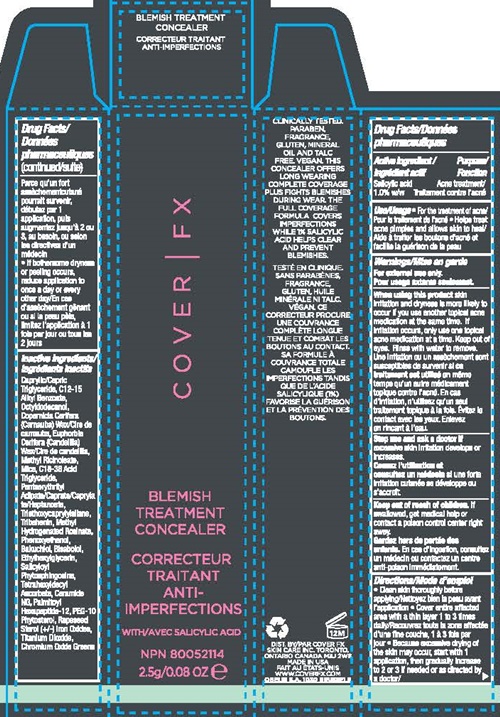
PRINCIPAL DISPLAY PANEL
COVER l FX
BLEMISH TREATMENT CONCEALER
2.5g/0.08 OZ e NPN 80052114
CLINICALLY TESTED, PARABEN, FRAGRANCE, GLUTEN, MINERAL OIL AND TALC FREE, VEGAN
THIS CONCEALER OFFERS LONG WEARING COMPLETE COVERAGE PLUS FIGHTS BLEMISHES DURING WEAR. THE FULL COVERAGE FORMULA COVERS IMPERFECTIONS WHILE 1% SALICYLIC ACID HELPS CLEAR AND PREVENT BLEMISHES.
DIST. BY/PAR
COVER FX SKIN CARE INC
TORONTO, ONTARIO CANADA M3J2W8
MADE IN USA
WWW.COVERFX.COM
RESPONSIBLE PERSONS:- OBELIS S.A., 1030 BRUSSELS