NDC Code(s) : 68382-720-06, 68382-720-16, 68382-720-01, 68382-720-05, 68382-720-10, 68382-720-30, 68382-720-77
Packager : Zydus Pharmaceuticals USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| budesonidebudesonide CAPSULE, COATED PELLETS | |||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Zydus Pharmaceuticals USA Inc.(156861945) |
| REGISTRANT - Zydus Pharmaceuticals USA Inc.(156861945) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Zydus Lifesciences Limited | 863362789 | ANALYSIS(68382-720), MANUFACTURE(68382-720) | |
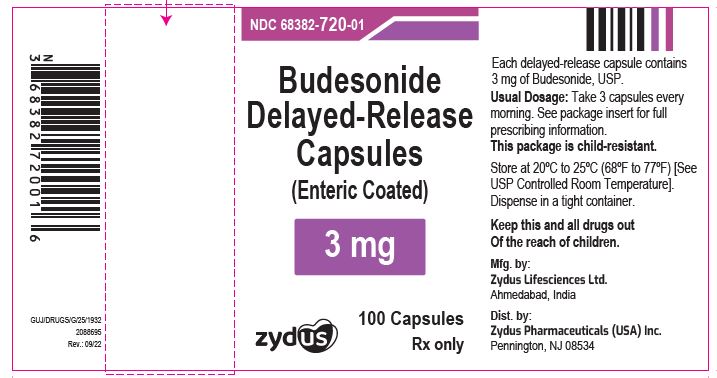
PRINCIPAL DISPLAY PANEL
NDC 68382-720-01 in bottle of 100 capsules
Budesonide Delayed-Release capsules (Enteric Coated) 3 mg
Rx only