NDC Code(s) : 68599-5401-2, 68599-5401-1, 68599-5401-3, 68599-5401-7, 68599-5401-4, 68599-5401-8, 68599-5401-5, 68599-5401-9, 68599-5401-6, 68599-5401-0
Packager : McKesson Medical-Surgical
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Antiseptic Skin CleanserCHLORHEXIDINE GLUCONATE SOLUTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - McKesson Medical-Surgical(023904428) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| XTTRIUM LABORATORIES, INC. | 007470579 | manufacture(68599-5401) | |
PRINCIPAL DISPLAY PANEL
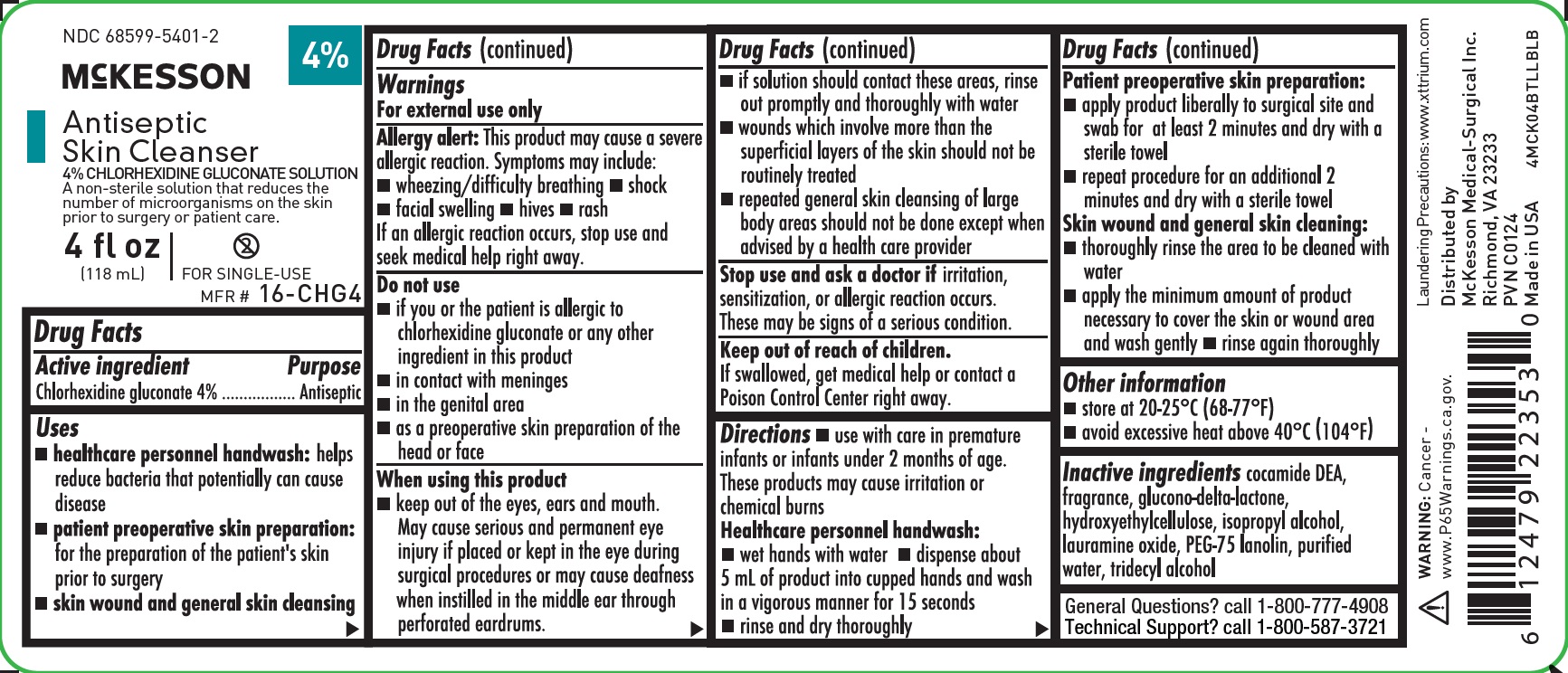
NDC 68599-5401-2
4%
McKESSON
AntisepticSkin Cleanser
4% CHLORHEXIDINE GLUCONATE SOLUTION
Reduces the number of microorganisms on the skin prior to surgery or patient care.
4 fl oz
(118 mL)
DO NOT REUSE
MFR #
16-CHG4
PRINCIPAL DISPLAY PANEL
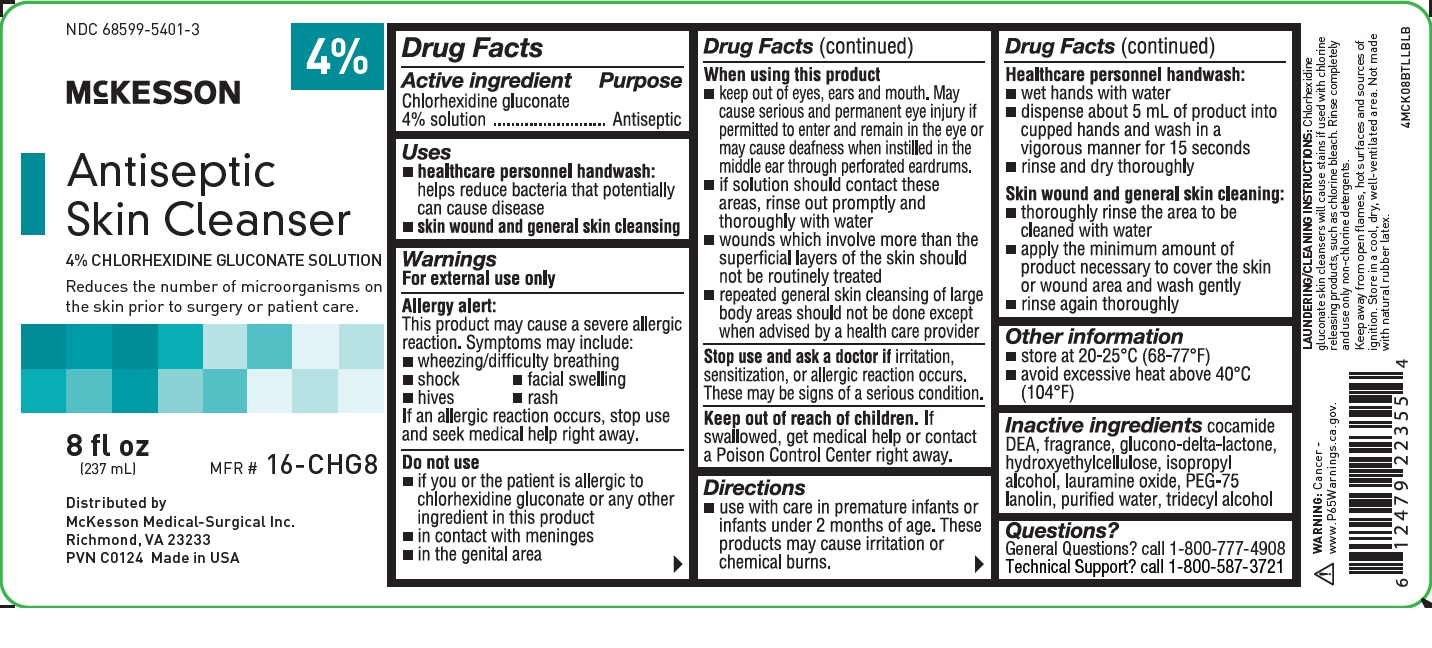
NDC 68599-5401-3
4%
McKESSON
Antiseptic Skin Cleanser
4% CHLORHEXIDINE GLUCONATE SOLUTION
Reduces the number of microorganisms on the skin prior to surgery or patient care.
8 fl oz
(237 mL)
MFR # 16-CHG8
PRINCIPAL DISPLAY PANEL
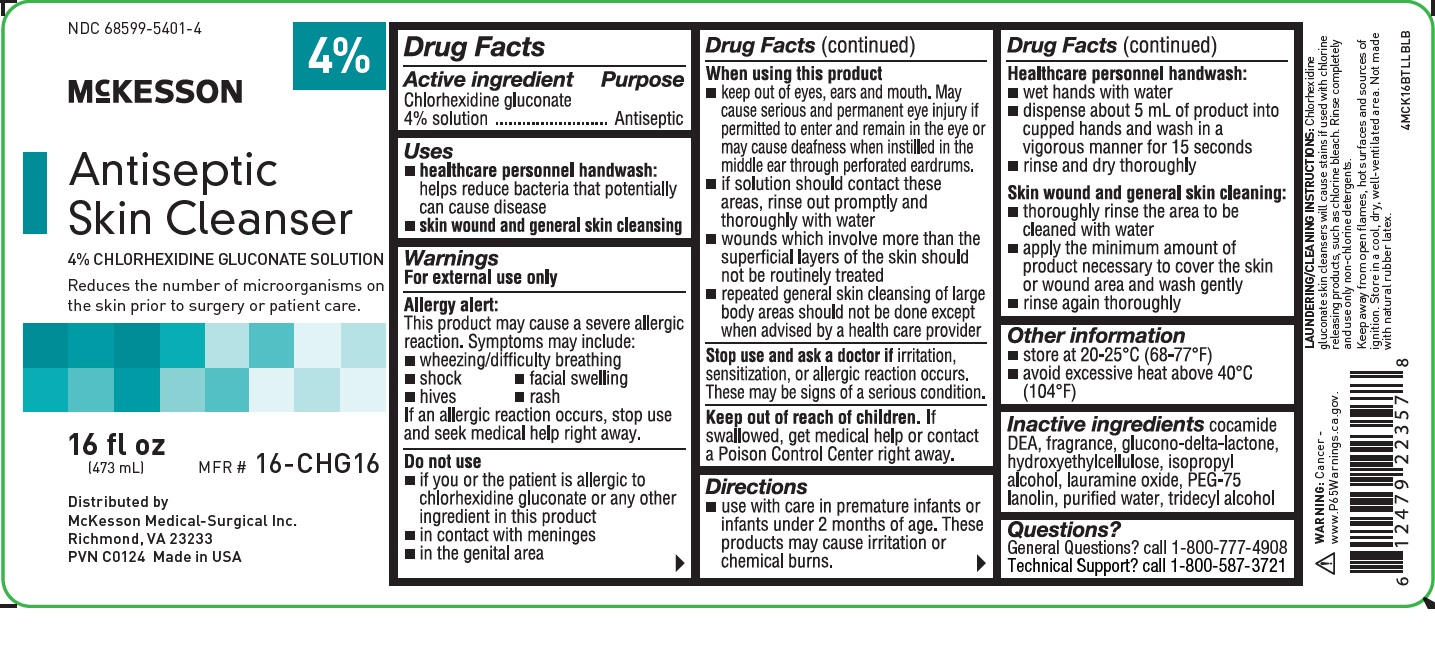
NDC 68599-5401-4
4%
McKESSON
Antiseptic Skin Cleanser
4% CHLORHEXIDINE GLUCONATE SOLUTION
Reduces the number of microorganisms on the skin prior to surgery or patient care.
16 fl oz
(473 mL)
MFR # 16-CHG16
PRINCIPAL DISPLAY PANEL
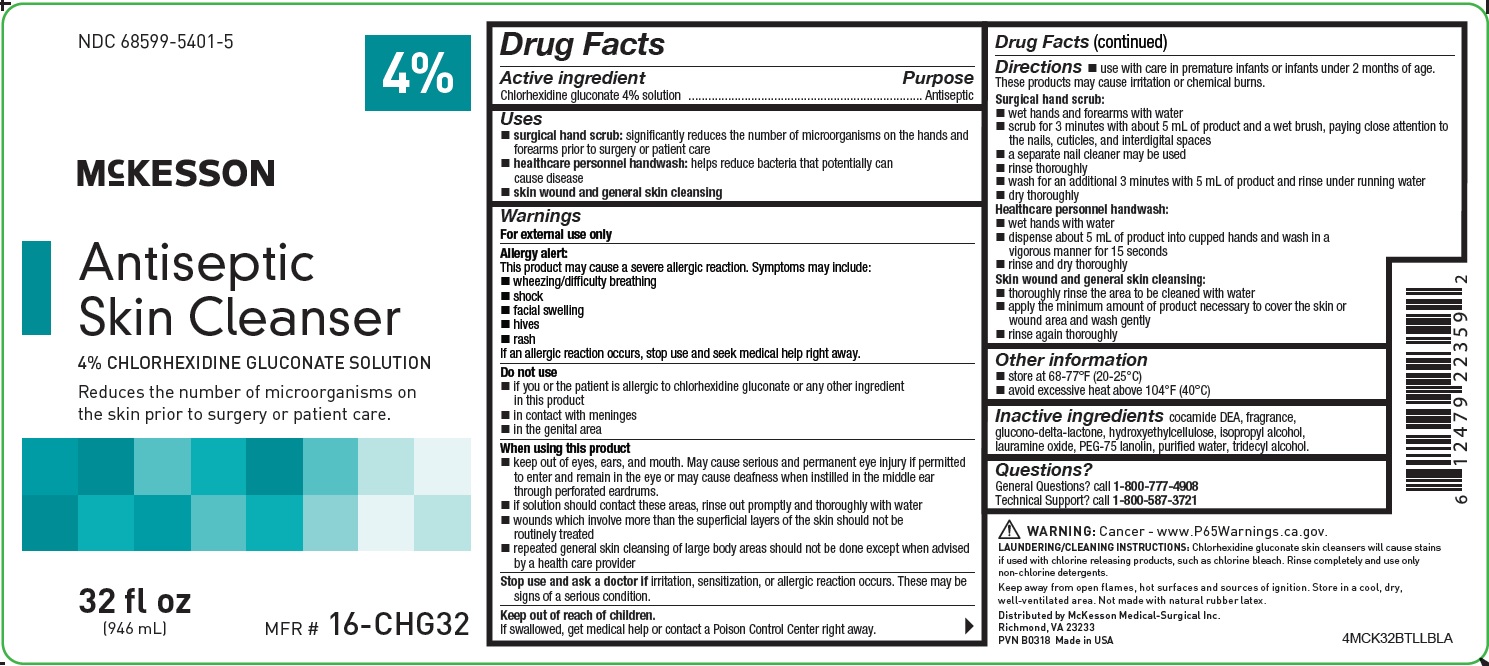
NDC 68599-5401-5
4%
McKESSON
Antiseptic Skin Cleanser
4% CHLORHEXIDINE GLUCONATE SOLUTION
Reduces the number of microorganisms on the skin prior to surgery or patient care.
32 fl oz
(946 mL)
MFR #
16-CHG32
PRINCIPAL DISPLAY PANEL
NDC 68599-5401-6
McKESSON
Antiseptic Skin Cleanser
4% CHLORHEXIDINE GLUCONATE SOLUTION
Reduces the number of microorganisms on the skin prior to surgery or patient care.
1 gal
(3.8 L)
MFR # 16-CHGGL










