NDC Code(s) : 68621-0027-1, 68621-0028-1, 68621-0029-1
Packager : Catalent Germany Schorndorf GmbH
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Jadenudeferasirox GRANULE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Jadenudeferasirox GRANULE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Jadenudeferasirox GRANULE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
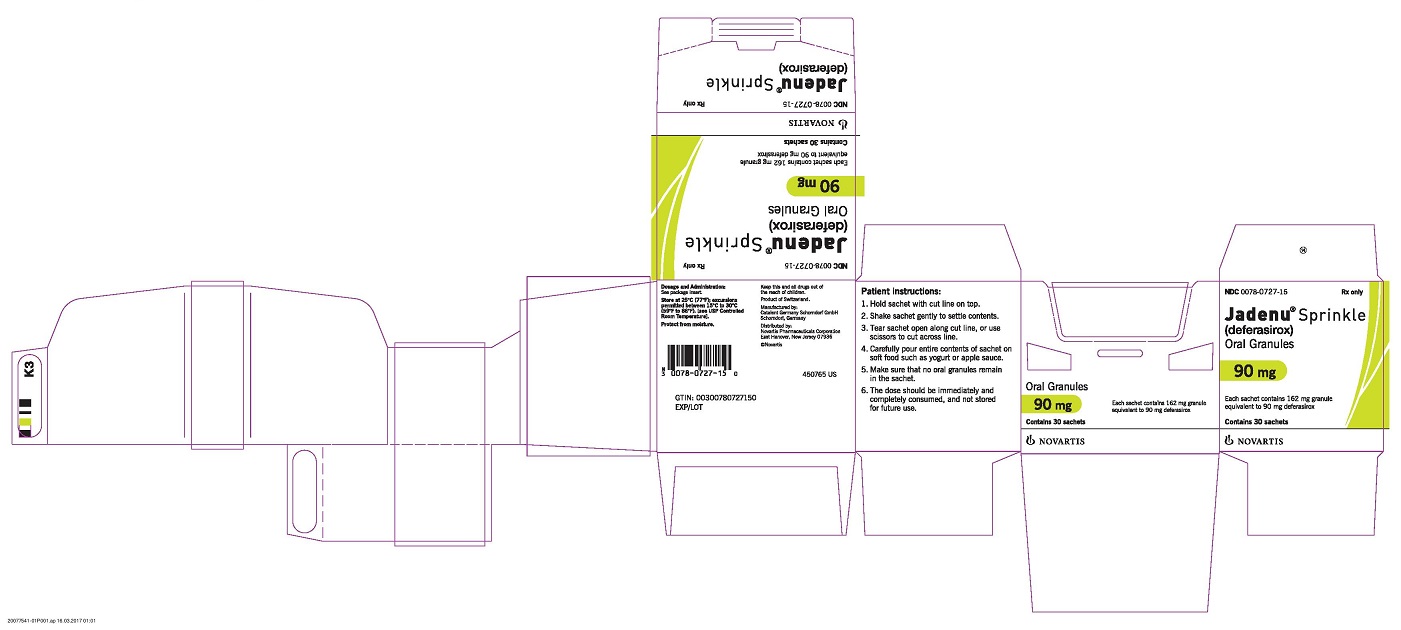
PRINCIPAL DISPLAY PANEL
Package Label – 90 mg
Rx Only NDC 0078-0727-15
Jadenu ® Sprinkle (deferasirox)
Oral Granules
90 mg
Each sachet contains 162 mg granule equivalent to 90 mg deferasirox
Contains 30 sachets
Novartis
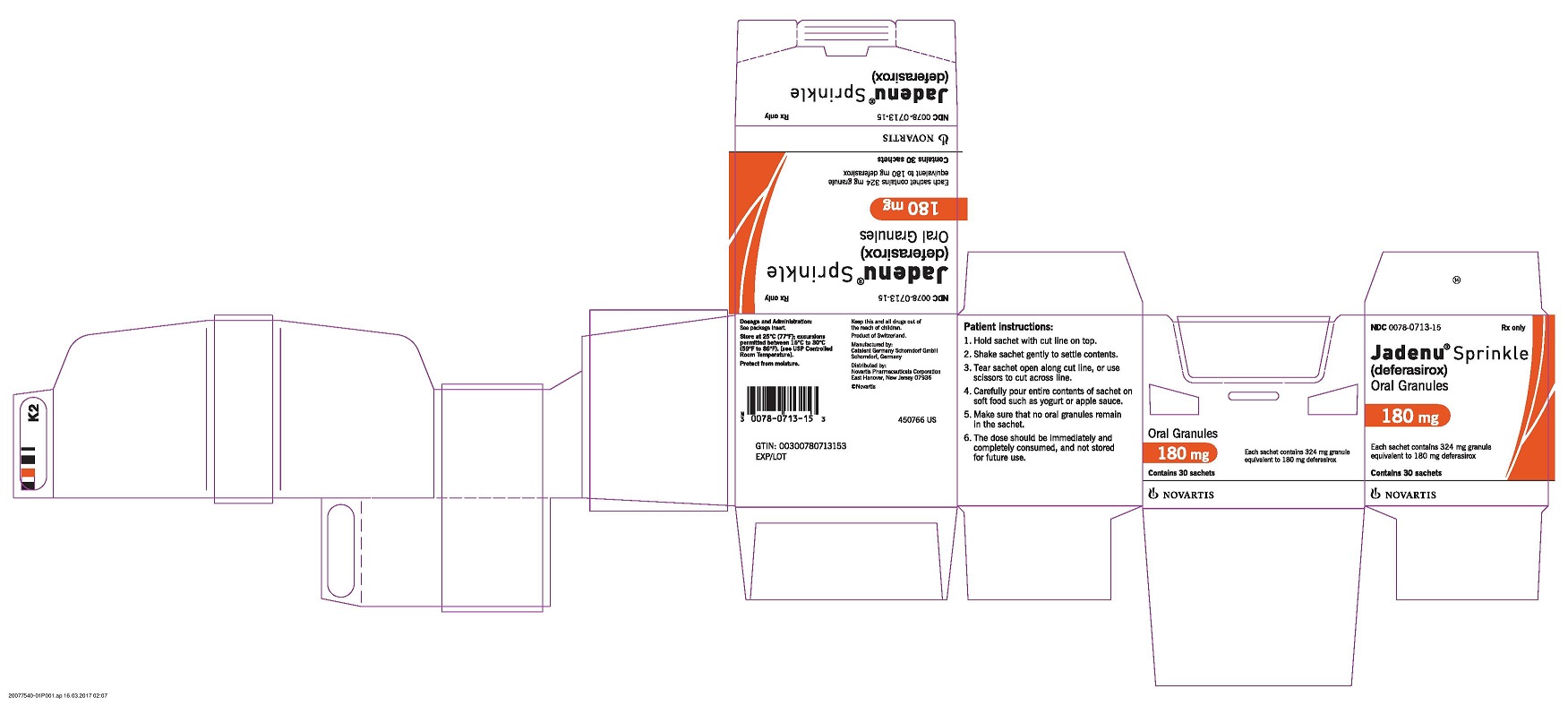
PRINCIPAL DISPLAY PANEL
Package Label – 180 mg
Rx Only NDC 0078-0713-15
Jadenu ® Sprinkle (deferasirox)
Oral Granules
180 mg
Each sachet contains 324 mg granule equivalent to 180 mg deferasirox
Contains 30 sachets
Novartis
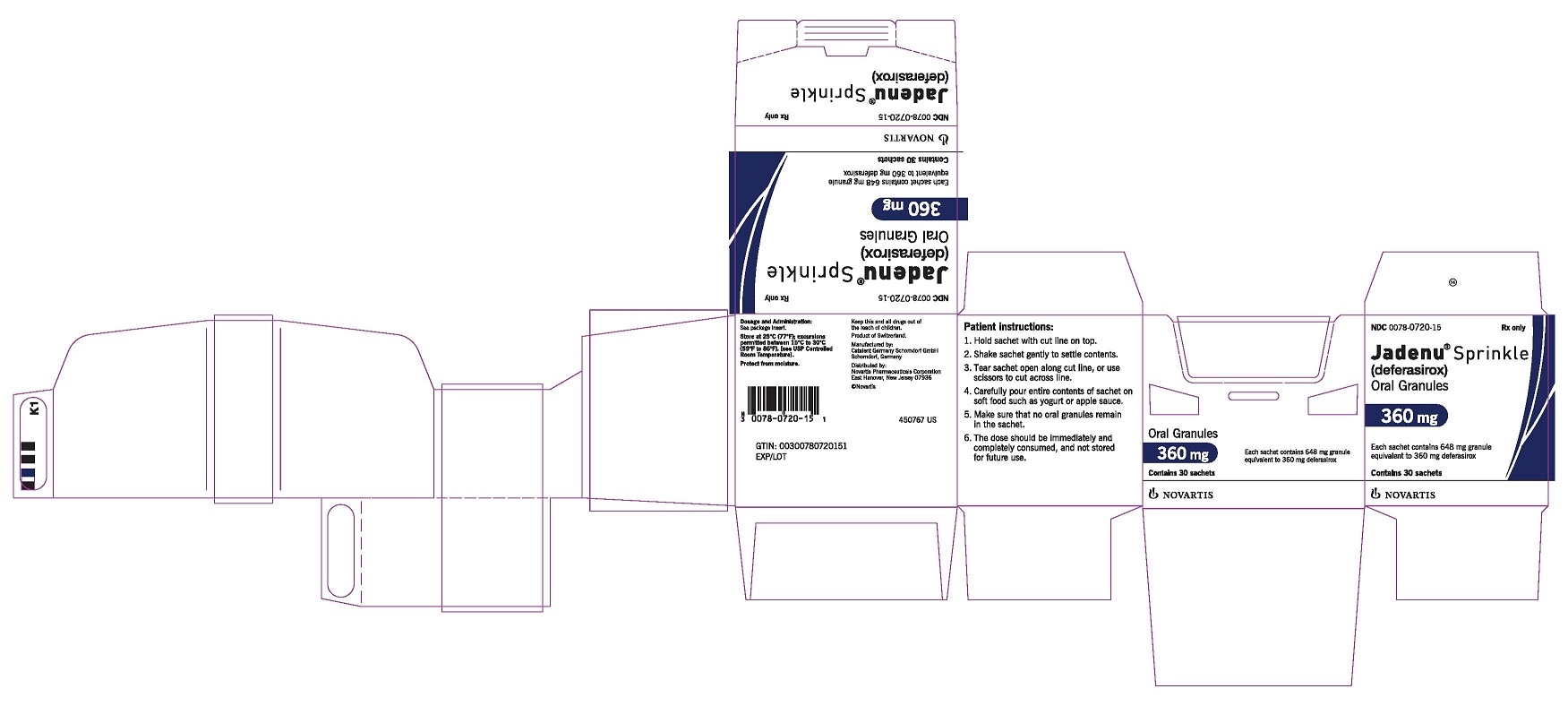
PRINCIPAL DISPLAY PANEL
Package Label – 360 mg
Rx Only NDC 0078-0720-15
Jadenu ® Sprinkle (deferasirox)
Oral Granules
360 mg
Each sachet contains 648 mg granule equivalent to 360 mg deferasirox
Contains 30 sachets
Novartis
PRINCIPAL DISPLAY PANEL
SHIIPING LABEL
Product: Jadenu GRU 90mg ALU (X30) US
Material code: 736126
Batch:
MFD:
ExP:
Quantity: 60 finished packs per shipper
NSC: 9A
Tempcontrol: Transport and intermediate storage not above +30ºC
Local Material: PCD460429CATALENT, CH
Handling Unite ID:
Catalent Order Number:
Customer PO Number:
Customer: Novartis Pharma

PRINCIPAL DISPLAY PANEL
SHIPPING LABEL
JADENU GRU 180mg ALU (X30) US
Material Code: 736127
Batch:
MFD:
EXP:
Quantity: 60 finished packs per shipper
NSC: 9A
Tempcontrol: Transport and Intermediate storage not above +30ºC
Local Material: PCD460432CATALENT, CH
Handling Unit iD:
Catalent Order number:
Customer PO Number:

PRINCIPAL DISPLAY PANEL
SHIPPING LABEL
Product: Jadenu GRU 360mg ALU (X30) US
Material Code: 736128
Batch:
MFD:
Quantity: 60 finished packs per shipper
NSC:9A
Tempcontrol: Transport and intermediate storage not above +30ºC
Local Material: PCD460433CATALENT,CH
Handling Unit ID:
Catalent Order number:
Customer PO number:

PRINCIPAL DISPLAY PANEL
PALLET LABEL
Product: Jadenu GRU 90mg ALU (X30) US
Novartis ESO PO:
Batch:
Material Code:
Customer PO:
MFD:
Delivery:
EXP:
SHIPPER:
Catalent Pharma Solutions GmbH
Riedstrabe 1
6330 Cham
Switzerland
Intermediate:
Planzer Transport AG
C/O Crossdocking
Salinenstrabe 63
4133 Pratteln
Switzerland
Consignee:
Shipping Marks:
Local Material: PCD460429CATALENT, CH
Handling Unit ID:
Quantity: 10180 finished packs per pallet
Net weight:
NSC: 9A
Gross Weight:
Tempcontrol: Transport and Intermediate storage not above +30ºC

PRINCIPAL DISPLAY PANEL
PALLET LABEL
Product: Jadenu GRU 180mg ALU (X30) US
Novartis ESO PO:
Batch:
Material Code: 736127
Customer PO:
MFD:
Delivery:
EXP:
Shipper:
Catalent Pharma Solutions GmbH
Riedstrabe 1
6330 Cham
Switzerland
Intermediate:
Planzer Transport AG
C/O Crossdocking
Salinenstrabe 63
4133 Pratteln
Switzerland
Consignee:
Shipping Marks:
Local Material: PCD46043CATALENT, CH
Handling Unit ID:
Quantity: 180 finished packs per pallet
Net Weight:
NSC: 9A
Gross Weight:
Tempcontrol: Transport and intermediate storage not above +30ºC

PRINCIPAL DISPLAY PANEL
PALLET LABEL
Product: Jadenu GRU 360mg ALU (X30) US
Novartis ESO PO:
Batch:
Material Code:
Customer PO:
MFD:
Delivery:
EXP:
Shipper:
Catalent Pharma Solutions, GmbH
Riedstrabe 1
6330 Cham
Switzerland
Intermediate:
Planzer Transport AG
C/O Crossdocking
Salinenstrabe 63
4133 Pratteln
Switzerland
Consignee:
Shipping Marks:
Local Material: PCD460433CATALENT,CH
Handling Unit ID:
Quantity: 1080 finished packs per pallet
Net weight:
Gross weight:
NSC: 9A
Tempcontrol: Transport and intermediate storage not above +30ºC










