NDC Code(s) : 68788-6990-1, 68788-6990-4
Packager : Preferred Pharmaceuticals Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Clotrimazole and Betamethasone DipropionateClotrimazole and Betamethasone Dipropionate CREAM | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LABELER - Preferred Pharmaceuticals Inc.(791119022) |
| REGISTRANT - Preferred Pharmaceuticals Inc.(791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Preferred Pharmaceuticals Inc. | 791119022 | RELABEL(68788-6990) | |
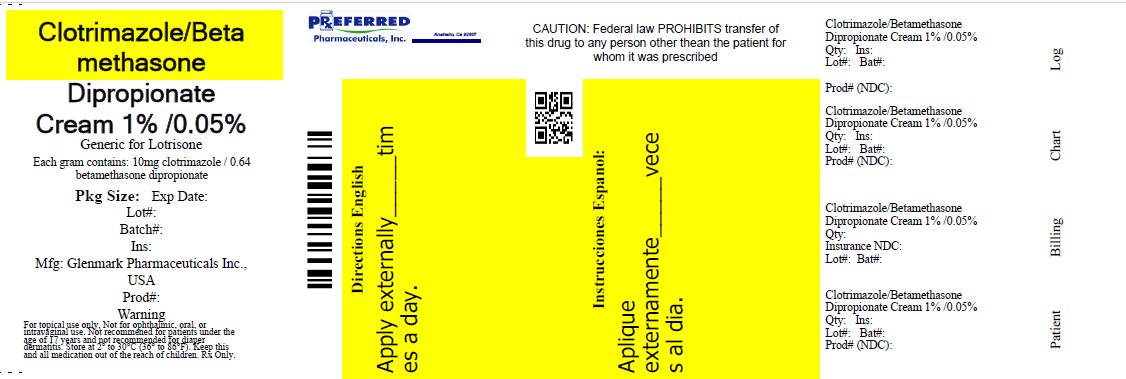
PRINCIPAL DISPLAY PANEL
NDC 68788-6990
Clotrimazole and Betamethasone Dipropionate Cream USP, 1%/0.05% (base)
FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE. NOT RECOMMENDED FOR PATIENTS UNDER THE AGE OF 17 YEARS AND NOT RECOMMENDED FOR DIAPER DERMATITIS.