NDC Code(s) : 68788-8400-5
Packager : Preferred Pharmaceuticals Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Ketorolac Tromethamineketorolac tromethamine SOLUTION/ DROPS | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Preferred Pharmaceuticals Inc.(791119022) |
| REGISTRANT - Preferred Pharmaceuticals Inc.(791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Preferred Pharmaceuticals Inc. | 791119022 | RELABEL(68788-8400) | |
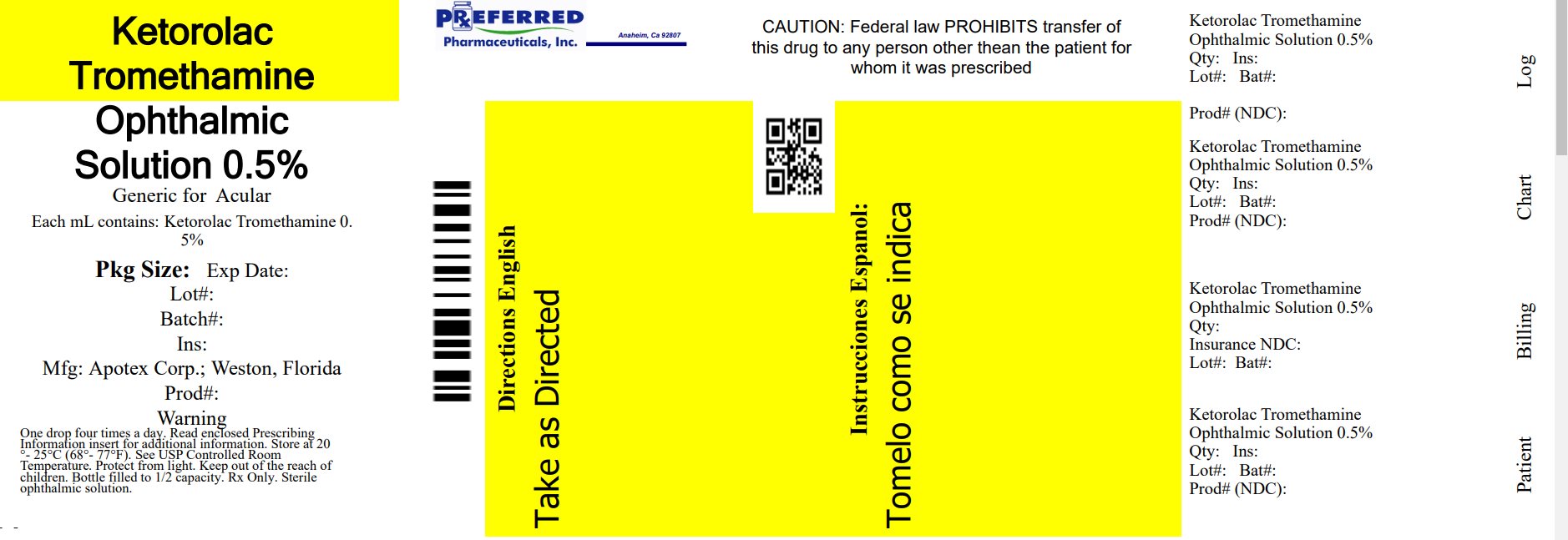
PRINCIPAL DISPLAY PANEL
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
PRINCIPAL DISPLAY PANEL - 0.5% BOTTLE LABEL
APOTEX CORP. NDC 68788-8400-5
Relabeled By: Preferred Pharmaceuticals Inc.
KETOROLAC TROMETHAMINE OPHTHALMIC SOLUTION 0.5%
Rx only
5 ml