NDC Code(s) : 68788-9969-3, 68788-9969-6, 68788-9969-9, 68788-9969-1, 68788-9324-3, 68788-9324-6, 68788-9324-9, 68788-9324-1
Packager : Preferred Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Nortriptyline HydrochlorideNortriptyline Hydrochloride CAPSULE | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Nortriptyline HydrochlorideNortriptyline Hydrochloride CAPSULE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 68788-9324
Nortriptyline
Hydrochloride
Capsules USP
25 mg*
*Each capsule contains:
Notriptyline Hydrochloride equivalent
to 25 mg Notriptyline
Usual Dosage: See package insert for
dosage and full prescribing information.
Dispense in a tight container, as definied
in the USP, with a child-resistant closure.
PHARMACIST: PLEASE DISPENSE WITH
MEDICATION GUIDE PROVIDED SEPARATELY.
Store at 20º - 25º C (68º - 77º F).
[See USP controlled room temperature.]
Manufactured By:
Watson Pharma Private Limited
Verna, Salcette Goa 403 722 INDIA
Code No. GO/DRUGS/741 173675
Distributed By: Watson Pharma, Inc.
Repackaged By: Preferred Pharmaceuticals Inc.

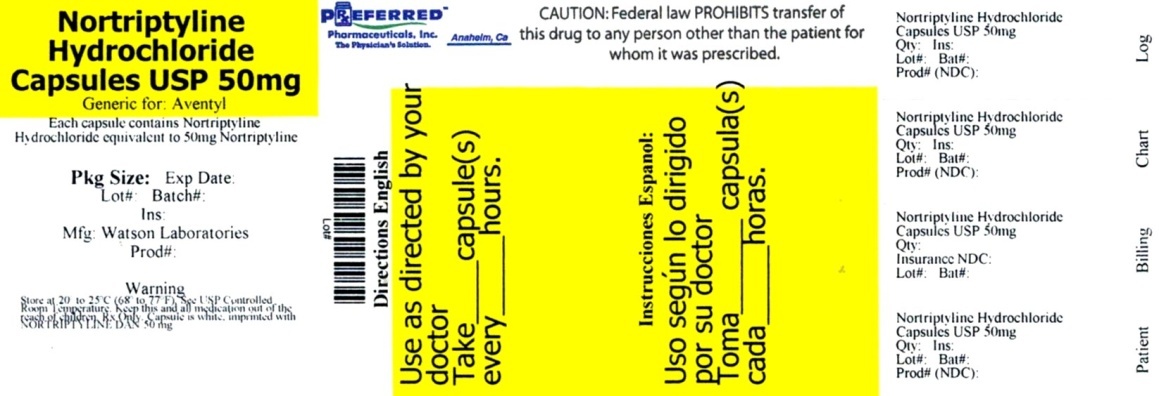
PRINCIPAL DISPLAY PANEL
NDC 68788-9969
Nortriptyline
Hydrochloride
Capsules USP
50 mg*
*Each capsule contains:
Notriptyline Hydrochloride equivalent
to 50 mg Notriptyline
Usual Dosage: See package insert for
dosage and full prescribing information.
Dispense in a tight container, as definied
in the USP, with a child-resistant closure.
PHARMACIST: PLEASE DISPENSE WITH
MEDICATION GUIDE PROVIDED SEPARATELY.
Store at 20º - 25º C (68º - 77º F).
[See USP controlled room temperature.]
Manufactured By:
Watson Pharma Private Limited
Verna, Salcette Goa 403 722 INDIA
Code No. GO/DRUGS/741 173677
Distributed By: Watson Pharma, Inc.
Repackaged By: Preferred Pharmaceuticals Inc.