NDC Code(s) : 68791-101-04
Packager : Royal Pharmaceuticals
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Derma-Smoothe/FSfluocinolone acetonide OIL | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Royal Pharmaceuticals(078374385) |
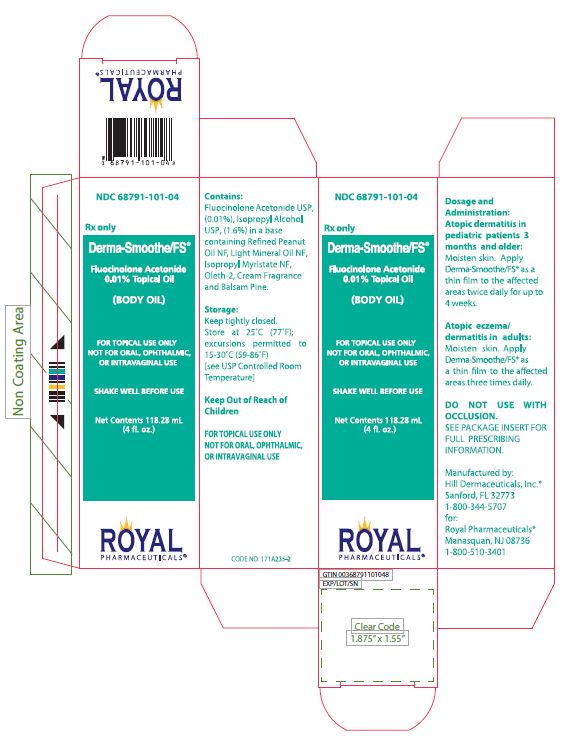
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 118.28 mL Bottle Label
NDC 68791-101-04
Rx only
Derma-Smoothe/FS®
Fluocinolone Acetonide
0.01% Topical Oil
(BODY OIL)
FOR TOPICAL USE ONLY
NOT FOR ORAL, OPHTHALMIC,
or INTRAVAGINAL USE
SHAKE WELL BEFORE USE
Net Contents 118.28 mL
(4 fL. oz.)
ROYAL
PHARMACEUTICALS®