NDC Code(s) : 68982-820-81, 68982-820-01, 68982-820-82, 68982-820-02, 68982-820-83, 68982-820-03, 68982-820-84, 68982-820-04, 68982-820-85, 68982-820-05, 68982-820-86, 68982-820-06
Packager : Octapharma USA Inc
Category : PLASMA DERIVATIVE
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Panzygaimmune globulin intravenous (human) SOLUTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Octapharma USA Inc(606121163) |
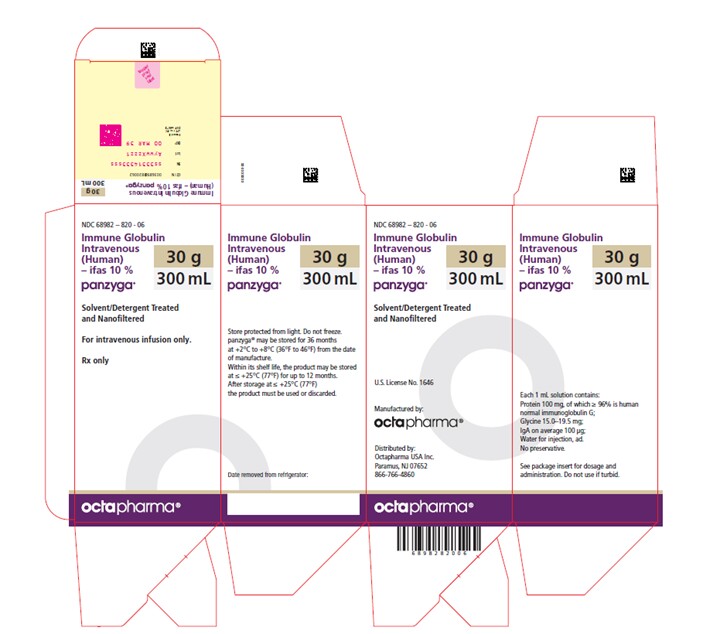
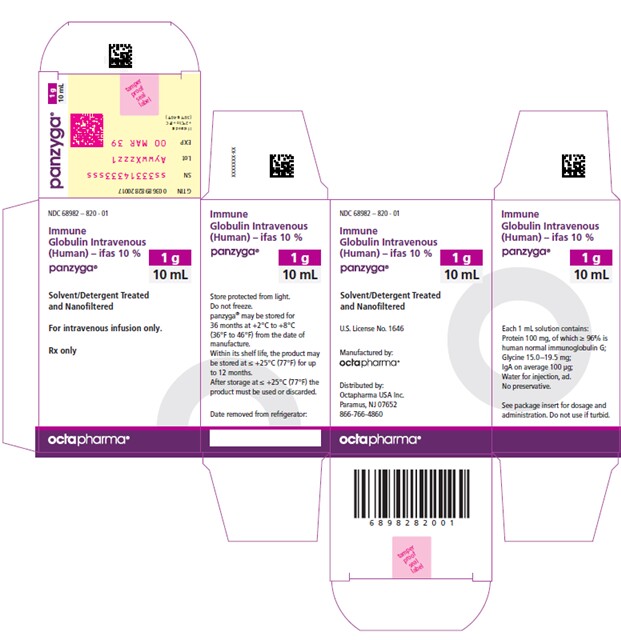
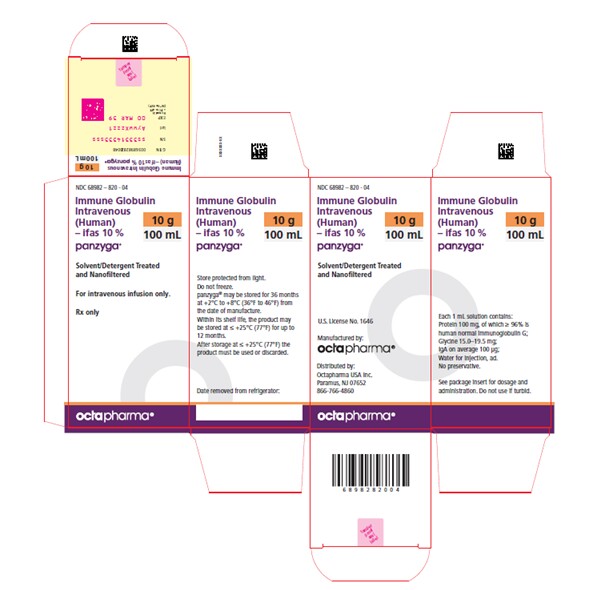
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL
Immune Globulin Intravenous (Human) - ifas, 10%
Panzyga
10 mL
NDC 68982-820-01
Panzyga
25 mL
NDC 68982-820-02

Panzyga
50 mL
NDC 68982-820-03

Panzyga
100 mL
NDC 68982-820-04
Panzyga
200 mL
NDC 68982-820-05

Panzyga
300 mL
NDC 68982-820-06