NDC Code(s) : 69067-100-08
Packager : Foxland Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AcetaminophenACETAMINOPHEN LIQUID | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
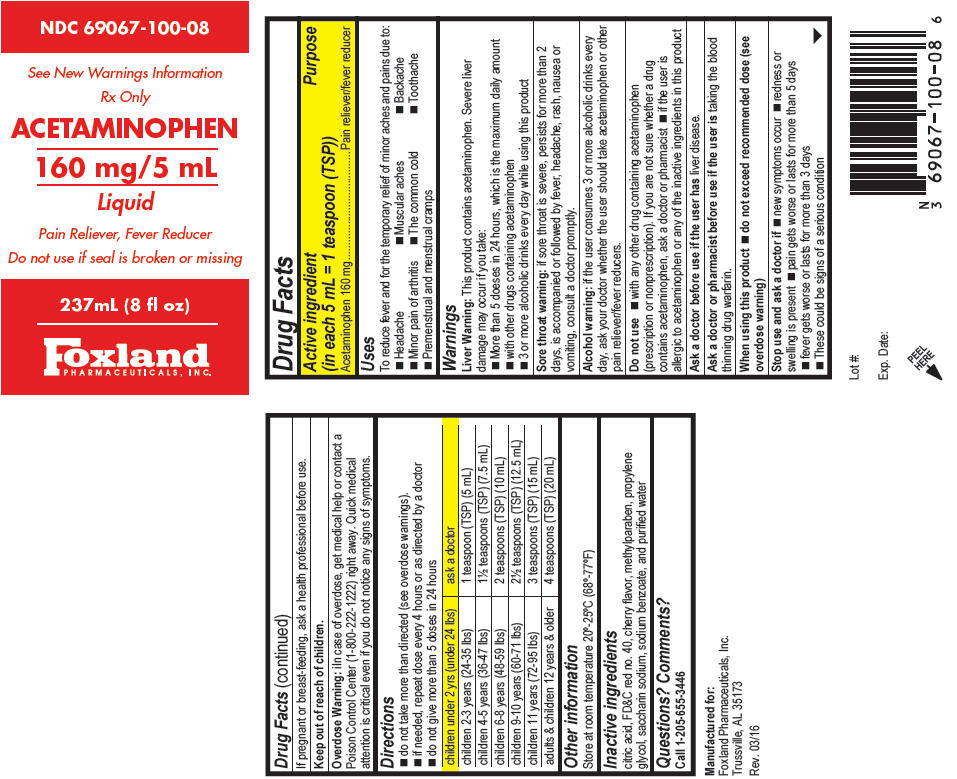
PRINCIPAL DISPLAY PANEL
NDC 69067-100-08
See New Warnings Information
Rx Only
ACETAMINOPHEN
160 mg/5 mL
Liquid
Pain Reliever, Fever Reducer
Do not use if seal is broken or missing
237mL (8 fl oz)
Foxland
PHARMACEUTICALS, INC.