NDC Code(s) : 69097-318-86, 69097-318-87, 69097-318-32, 69097-318-53, 69097-319-86, 69097-319-87, 69097-319-32, 69097-319-53, 69097-321-86, 69097-321-87, 69097-321-32, 69097-321-53
Packager : Cipla USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Budesonide Budesonide INHALANT | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Budesonide Budesonide INHALANT | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Budesonide Budesonide INHALANT | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Cipla USA Inc.(078719707) |
| REGISTRANT - Cipla USA Inc.(078719707) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Cipla Ltd. Goa | 650072015 | MANUFACTURE(69097-318, 69097-319, 69097-321) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Cipla Ltd. Indore | 918596409 | MANUFACTURE(69097-318, 69097-319, 69097-321) | |
PRINCIPAL DISPLAY PANEL
↑ Store Upright ↑
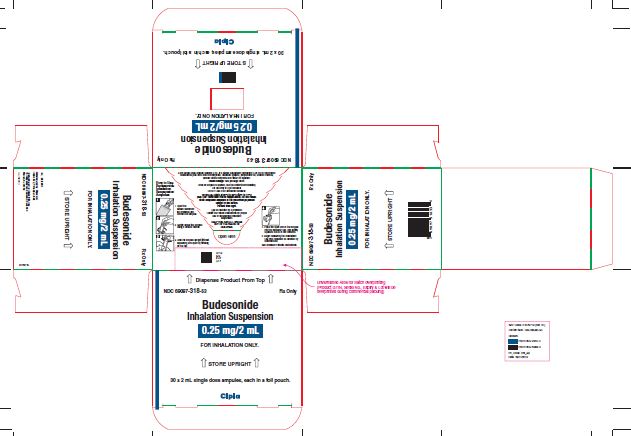
NDC 69097-318-32 Rx Only
Budesonide
Inhalation
Suspension
0.25 mg/2mL
FOR INHALATION ONLY.
Each single-dose ampule
delivers 2 mL of a sterile
suspension containing 0.25 mg
of micronized budesonide plus
citric acid monohydrate,
disodium edetate, polysorbate
80, sodium chloride, sodium
citrate dihydrate and water for
injection.
Usual Dosage: See package
insert. Shake gently using a
circular motion prior to use.
Once an ampule is opened,
use the contents immediately.
For use only in a jet nebulizer.
Do NOT use in an ultrasonic
nebulizer.
Store at 20°C to 25°C
(68°F to 77°F) [See USP
Controlled Room
Temperature]. Do not freeze.
Store unopened ampules in
the foil envelope placed
upright in the carton.
Protect from light.
1 envelope x one 2 mL
single-dose ampule
Cipla
Use as directed by a physician.
Follow the Patient Instructions for proper
use of budesonide inhalation suspension.
Keep out of reach of children.

↑ Dispense Product From Top ↑
NDC 69097-318-53 Rx ONLY
Budesonide
Inhalation
Suspension
0.25mg/2 mL
FOR INHALATION ONLY.
↑ STORE UPRIGHT ↑
30 x 2 mL single dose ampules, each in a foil pouch
Cipla
↑ Store Upright ↑
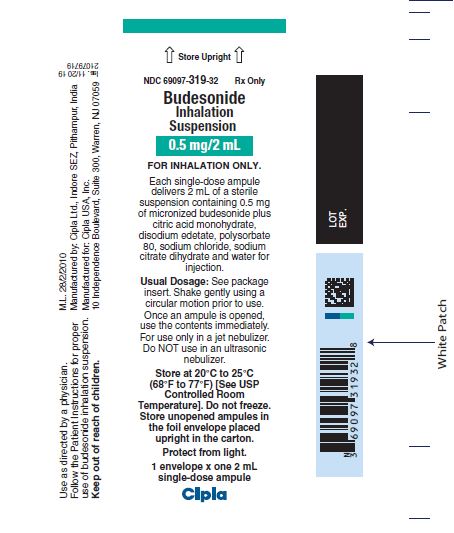
NDC 69097-319-32 Rx Only
Budesonide
Inhalation
Suspension
0.5 mg/2mL
FOR INHALATION ONLY.
Each single-dose ampule
delivers 2 mL of a sterile
suspension containing 0.5 mg
of micronized budesonide plus
citric acid monohydrate,
disodium edetate, polysorbate
80, sodium chloride, sodium
citrate dihydrate and water for
injection.
Usual Dosage: See package
insert. Shake gently using a
circular motion prior to use.
Once an ampule is opened,
use the contents immediately.
For use only in a jet nebulizer.
Do NOT use in an ultrasonic
nebulizer.
Store at 20°C to 25°C
(68°F to 77°F) [See USP
Controlled Room
Temperature]. Do not freeze.
Store unopened ampules in
the foil envelope placed
upright in the carton.
Protect from light.
1 envelope x one 2 mL
single-dose ampule
Cipla
0.5 mg/2 mL
Use as directed by a physician.
Follow the Patient Instructions for proper
use of budesonide inhalation suspension.
Keep out of reach of children.
↑ Dispense Product From Top ↑
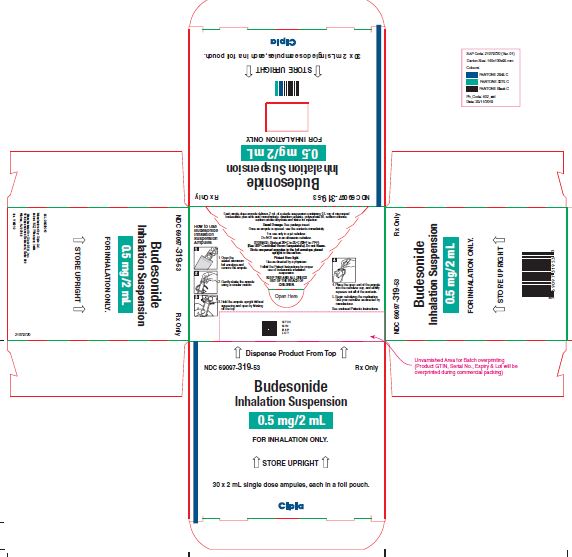
NDC 69097-319-53 Rx Only
Budesonide
Inhalation Suspension
0.5 mg/2mL
FOR INHALATION ONLY.
↑ STORE UPRIGHT ↑
30 x 2 mL single dose ampules, each in a foil pouch.
Cipla
↑ Store Upright ↑
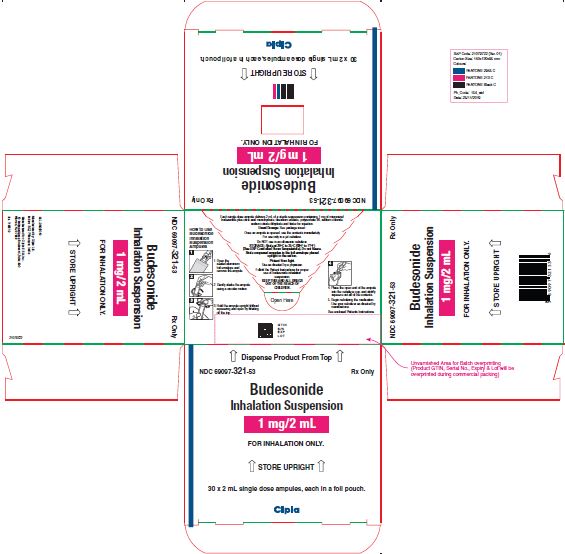
NDC 69097-321-32 Rx Only
Budesonide
Inhalation
Suspension
1 mg/2mL
FOR INHALATION ONLY.
Each single-dose ampule
delivers 2 mL of a sterile
suspension containing 1 mg
of micronized budesonide plus
citric acid monohydrate,
disodium edetate, polysorbate
80, sodium chloride, sodium
citrate dihydrate and water for
injection.
Usual Dosage: See package
insert. Shake gently using a
circular motion prior to use.
Once an ampule is opened,
use the contents immediately.
For use only in a jet nebulizer.
Do NOT use in an ultrasonic
nebulizer.
Store at 20°C to 25°C
(68°F to 77°F) [See USP
Controlled Room
Temperature]. Do not freeze.
Store unopened ampules in
the foil envelope placed
upright in the carton.
Protect from light.
1 envelope x one 2 mL
single-dose ampule
Cipla
0.5 mg/2 mL
Use as directed by a physician.
Follow the Patient Instructions for proper
use of budesonide inhalation suspension.
Keep out of reach of children.

↑ Dispense Product From Top ↑
NDC 69097-321-53 Rx Only
Budesonide
Inhalation Suspension
1 mg/2mL
FOR INHALATION ONLY.
↑ STORE UPRIGHT ↑
30 x 2 mL single dose ampules, each in a foil pouch.
Cipla
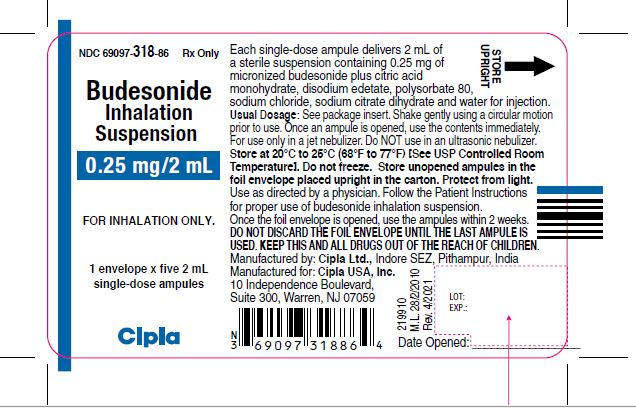
NDC 69097-318-86 Rx ONLY
Budesonide
Inhalation
Suspension
0.25 mg/2 mL
FOR INHALATION ONLY.
1 envelope x five 2 mL
Single-dose ampules
Cipla
NDC 69097-318-87 Rx ONLY
Budesonide
Inhalation
Suspension
0.25 mg/2 mL
FOR INHALATION ONLY.
↑ STORE UPRIGHT↑
30 single-dose
ampules per carton
Cipla

NDC 69097-319-86 Rx ONLY
Budesonide
Inhalation
Suspension
0.5 mg/2 mL
FOR INHALATION ONLY.
1 envelope x five 2 mL
Single-dose ampules
Cipla

NDC 69097-319-87 Rx ONLY
Budesonide
Inhalation
Suspension
0.5 mg/2 mL
FOR INHALATION ONLY.
↑ STORE UPRIGHT ↑
30 single-dose
ampules per carton
Cipla

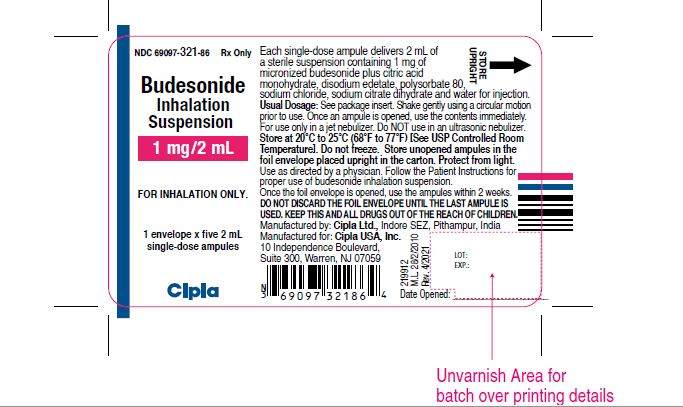
NDC 69097-321-86 Rx ONLY
Budesonide
Inhalation
Suspension
1 mg/2 mL
FOR INHALATION ONLY.
1 envelope x five 2 mL
Single-dose ampules
Cipla
NDC 69097-321-87 Rx ONLY
Budesonide
Inhalation
Suspension
1 mg/2 mL
FOR INHALATION ONLY.
↑ STORE UPRIGHT ↑
30 single-dose
ampules per carton
Cipla










