NDC Code(s) : 69097-503-27, 69097-503-31
Packager : Cipla USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Dihydroergotamine Mesylate Nasal Dihydroergotamine Mesylate Nasal SPRAY, METERED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Cipla USA Inc.(078719707) |
| REGISTRANT - Cipla USA Inc.(078719707) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Mipharm S.p.A., Italy | 514042399 | ANALYSIS(69097-503), LABEL(69097-503), MANUFACTURE(69097-503), PACK(69097-503) | |
PRINCIPAL DISPLAY PANEL
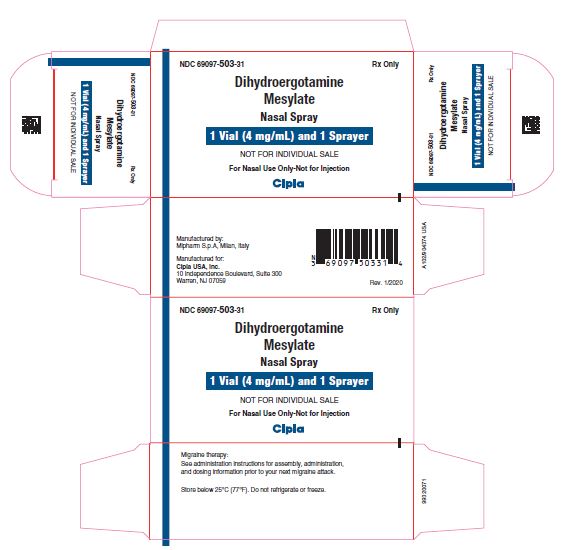
NDC 69097 503 31 Rx Only
Dihydroergotamine Mesylate
Nasal Spray
4 mg/mL (1 mL/Vial)
FOR NASAL USE ONLY
NOT FOR INJECTION
Cipla
 cipla-vial-label
cipla-vial-label
NDC 69097 503 31 Rx Only
Dihydroergotamine Mesylate
Nasal Spray
1 vial (4 mg/mL) and 1 Sprayer
NOT FOR INDIVIDUAL SALE
For Nasal Use Only-Not for Injection
Cipla
 cipla-inner-carton-label
cipla-inner-carton-label
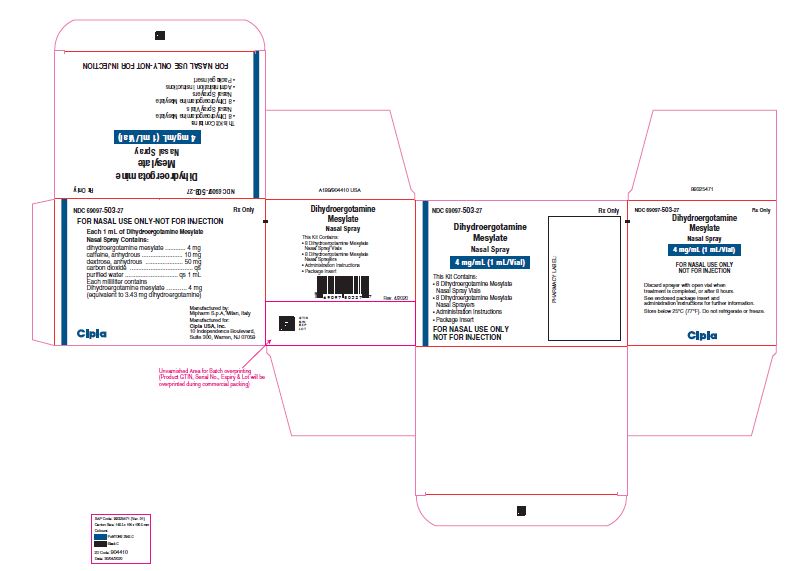
NDC 69097 503 27 Rx Only
Dihydroergotamine Mesylate
Nasal Spray
4 mg/mL (1 mL/Vial)
This Kit Contains:
- 8 Dihydroergotamine Mesylate Nasal Spray Vials
- 8 Dihydroergotamine Mesylate Nasal Sprayers
- Administration Instructions
- Package insert
FOR NASAL USE ONLY
NOT FOR INJECTION
Cipla
 cipla-outer-carton-label
cipla-outer-carton-label









