NDC Code(s) : 69097-550-53, 69097-560-53, 69097-570-53
Packager : Cipla USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Deferasirox Deferasirox GRANULE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Deferasirox Deferasirox GRANULE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Deferasirox Deferasirox GRANULE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Cipla USA Inc.(078719707) |
| REGISTRANT - Cipla USA Inc.(078719707) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Cipla Limited- Kurkumbh | 917066446 | API MANUFACTURE(69097-550, 69097-560, 69097-570), MANUFACTURE(69097-550, 69097-560, 69097-570) | |
PRINCIPAL DISPLAY PANEL
NDC 69097-550-53
Deferasirox Oral Granules, 90 mg
Rx Only
Cipla USA, Inc.
Carton Label (30 Sachets in 1 Carton)

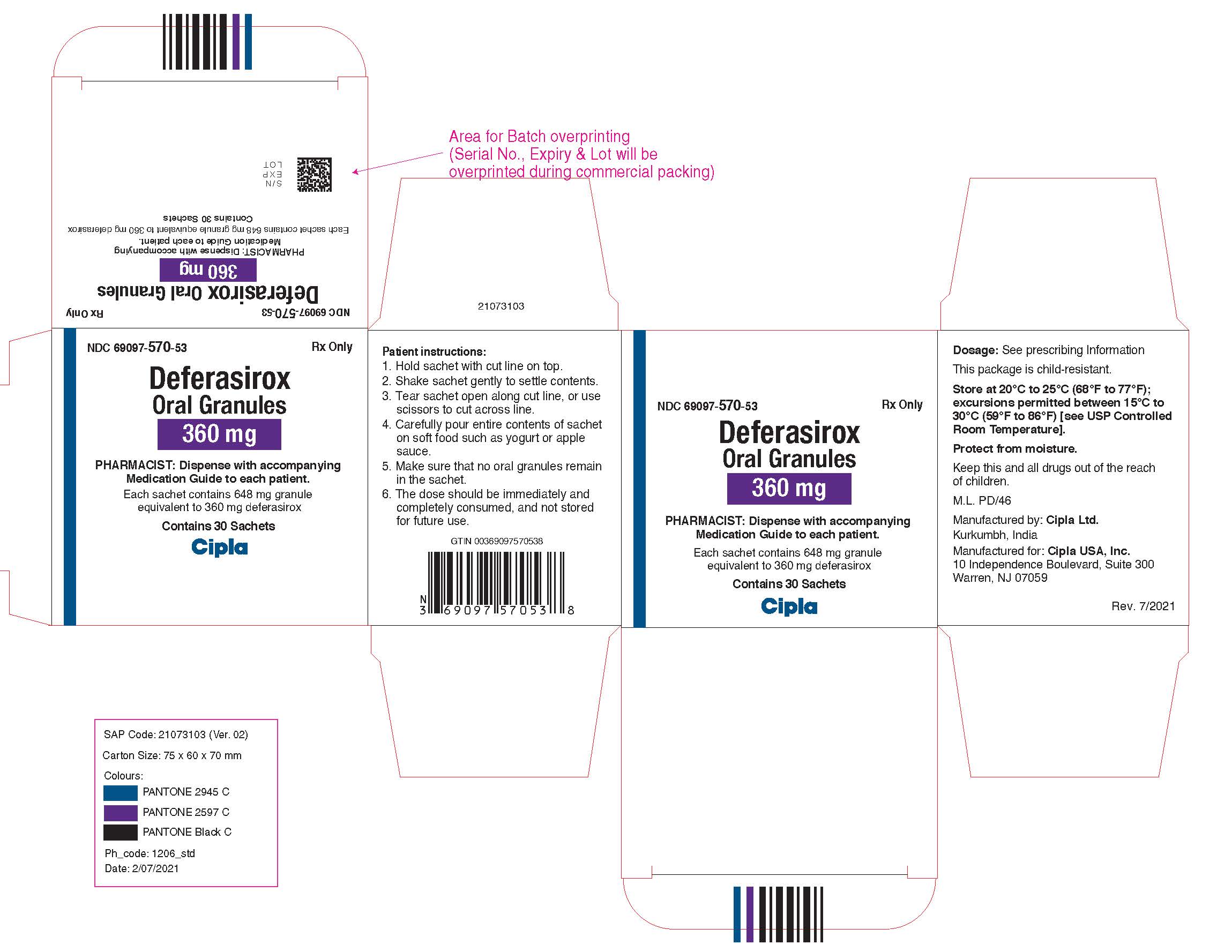
NDC 69097-560-53
Deferasirox Oral Granules, 180 mg
Rx Only
Cipla USA, Inc.
Carton Label (30 Sachets in 1 Carton)
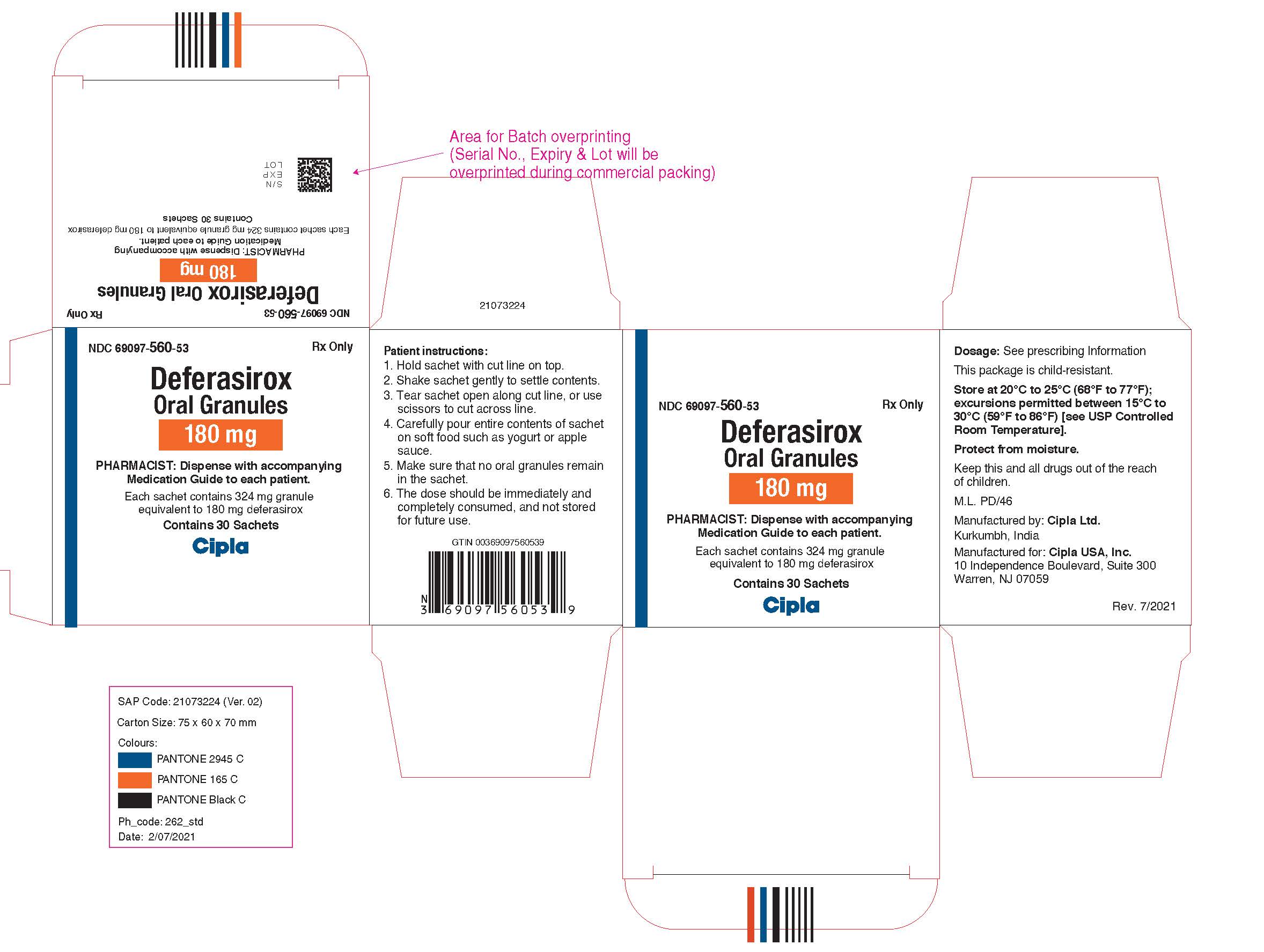
NDC 69097-570-53
Deferasirox Oral Granules, 360 mg
Rx Only
Cipla USA, Inc.
Carton Label (30 Sachets in 1 Carton)