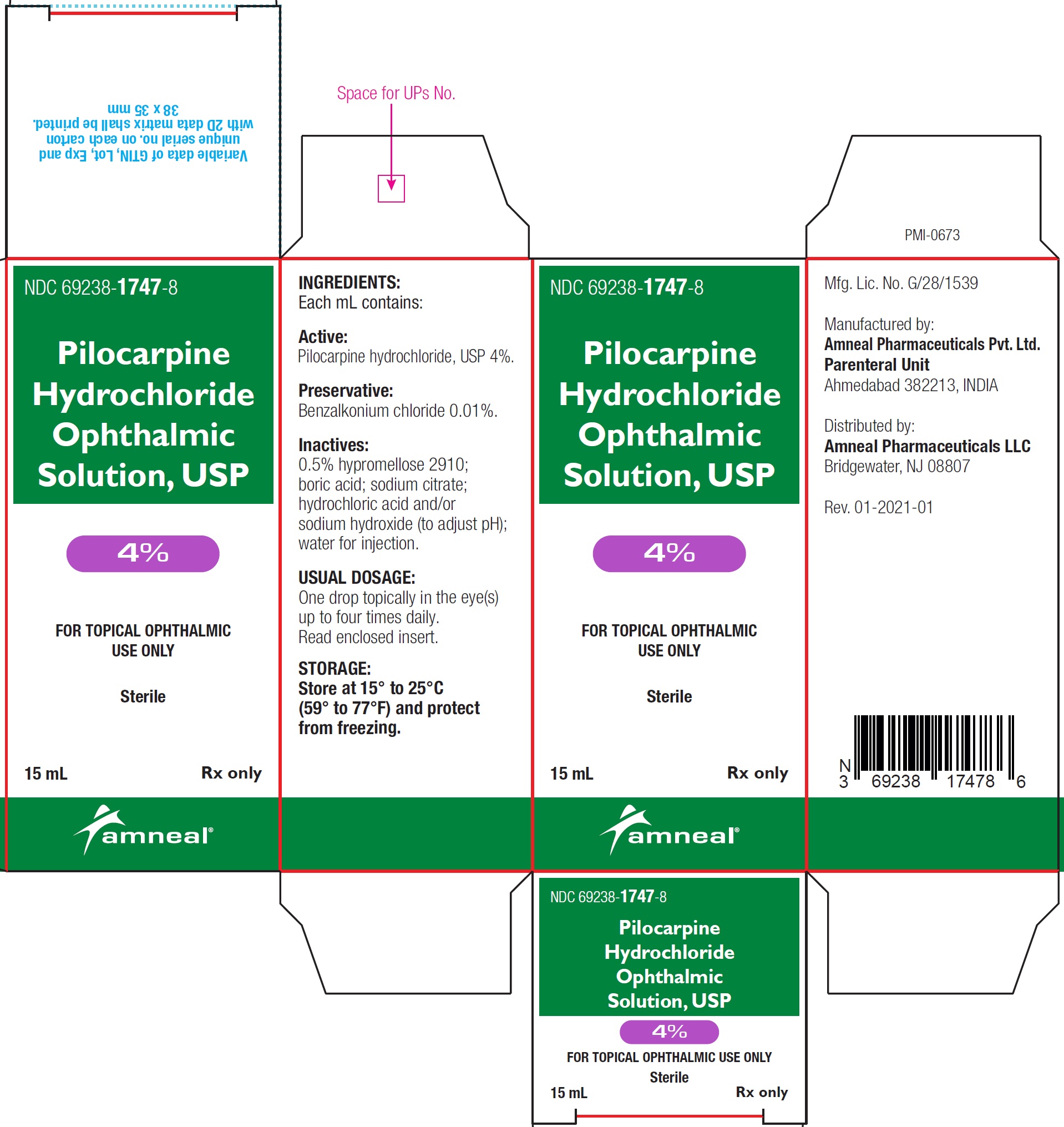
NDC Code(s) : 69238-1745-8, 69238-1746-8, 69238-1747-8
Packager : Amneal Pharmaceuticals NY LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Pilocarpine HydrochloridePilocarpine Hydrochloride SOLUTION/ DROPS | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Pilocarpine HydrochloridePilocarpine Hydrochloride SOLUTION/ DROPS | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Pilocarpine HydrochloridePilocarpine Hydrochloride SOLUTION/ DROPS | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Amneal Pharmaceuticals NY LLC(123797875) |
PRINCIPAL DISPLAY PANEL
NDC 69238-1745-8
Pilocarpine hydrochloride ophthalmic solution USP, 1%
Rx only
Amneal Pharmaceuticals LLC

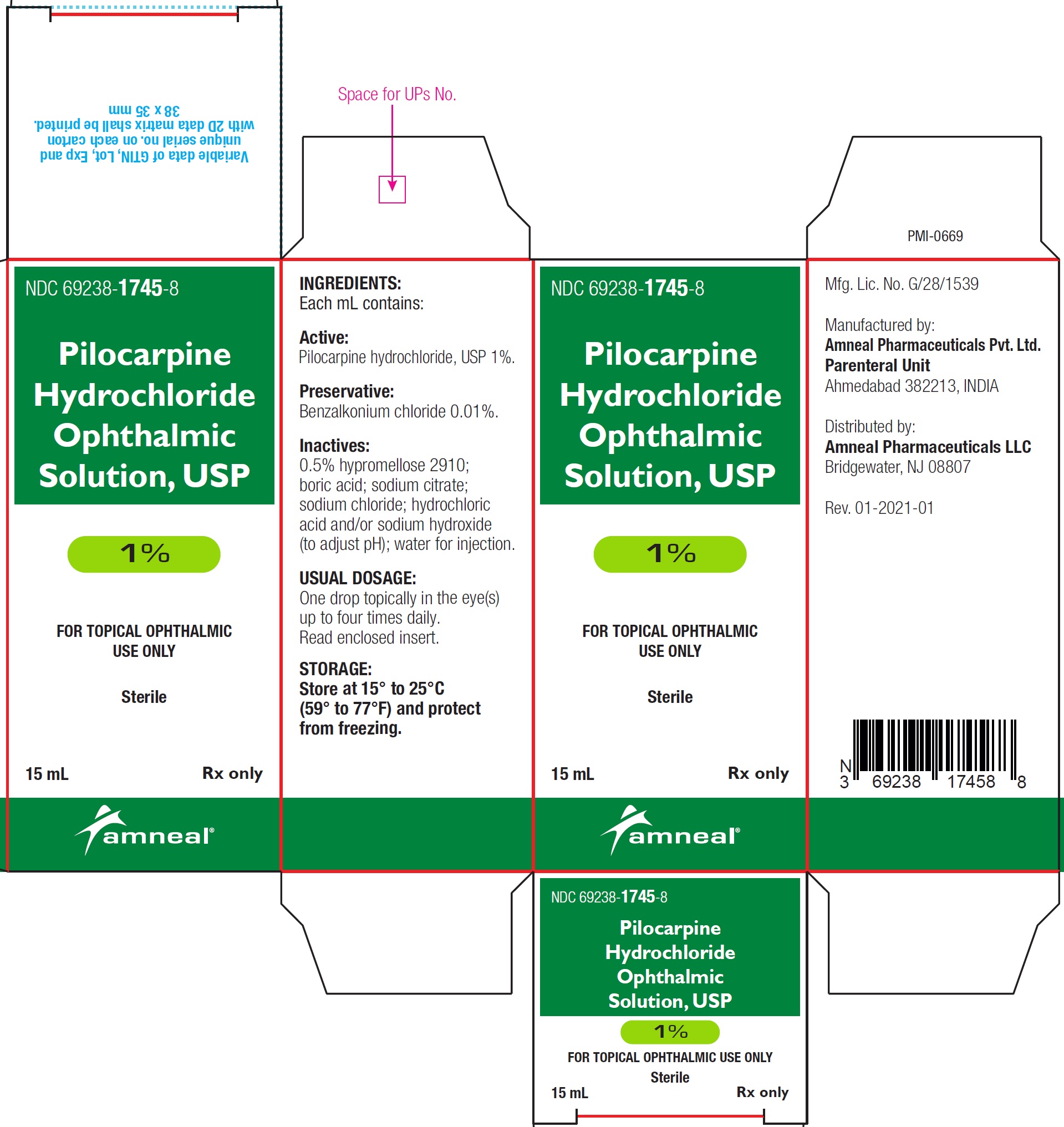
NDC 69238-1746-8
Pilocarpine hydrochloride ophthalmic solution USP, 2%
Rx only
Amneal Pharmaceuticals LLC

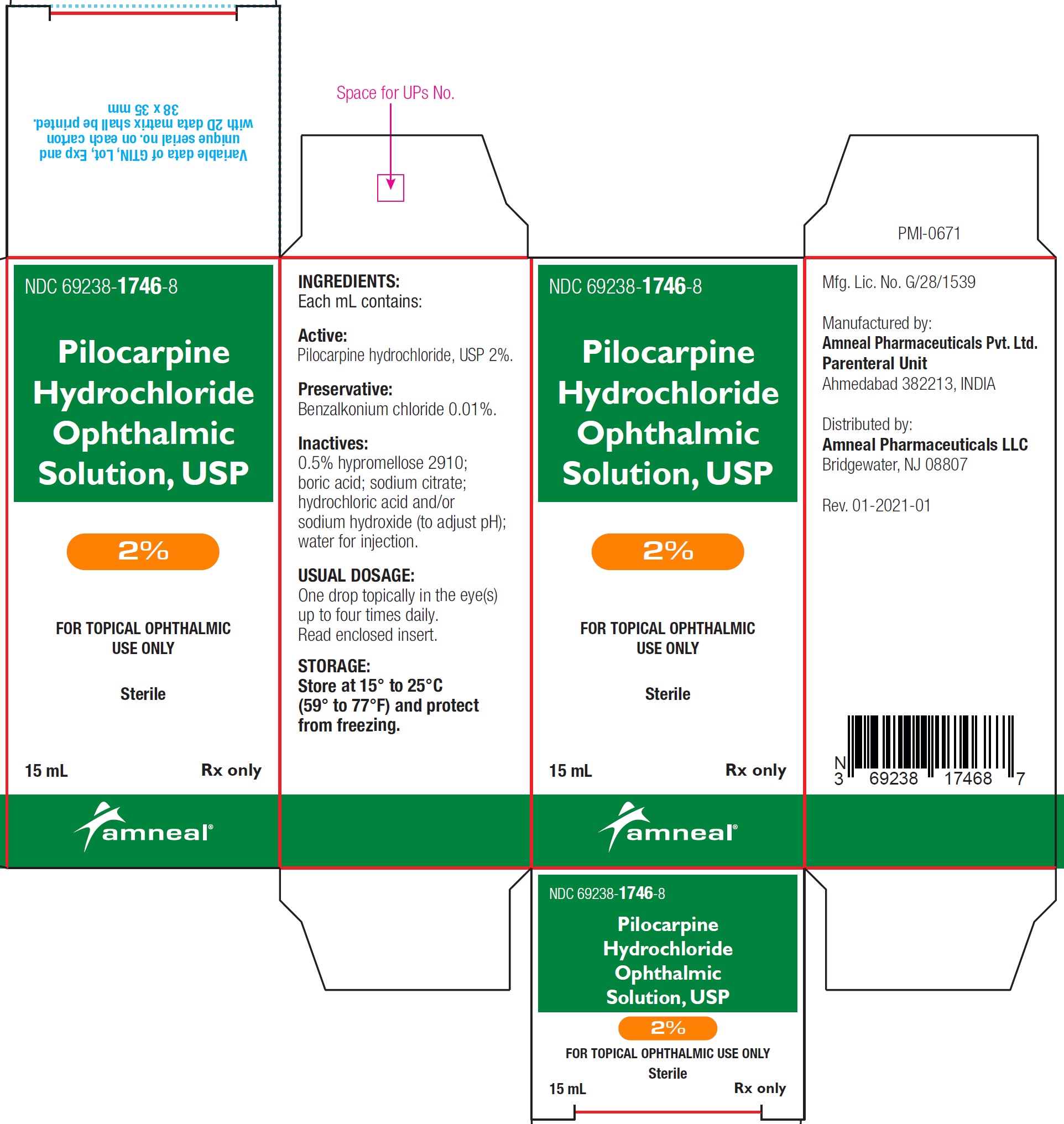
NDC 69238-1747-8
Pilocarpine hydrochloride ophthalmic solution USP, 4%
Rx only
Amneal Pharmaceuticals LLC