NDC Code(s) : 69292-015-35
Packager : Amici Pharmaceuticals LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ganciclovirganciclovir GEL | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
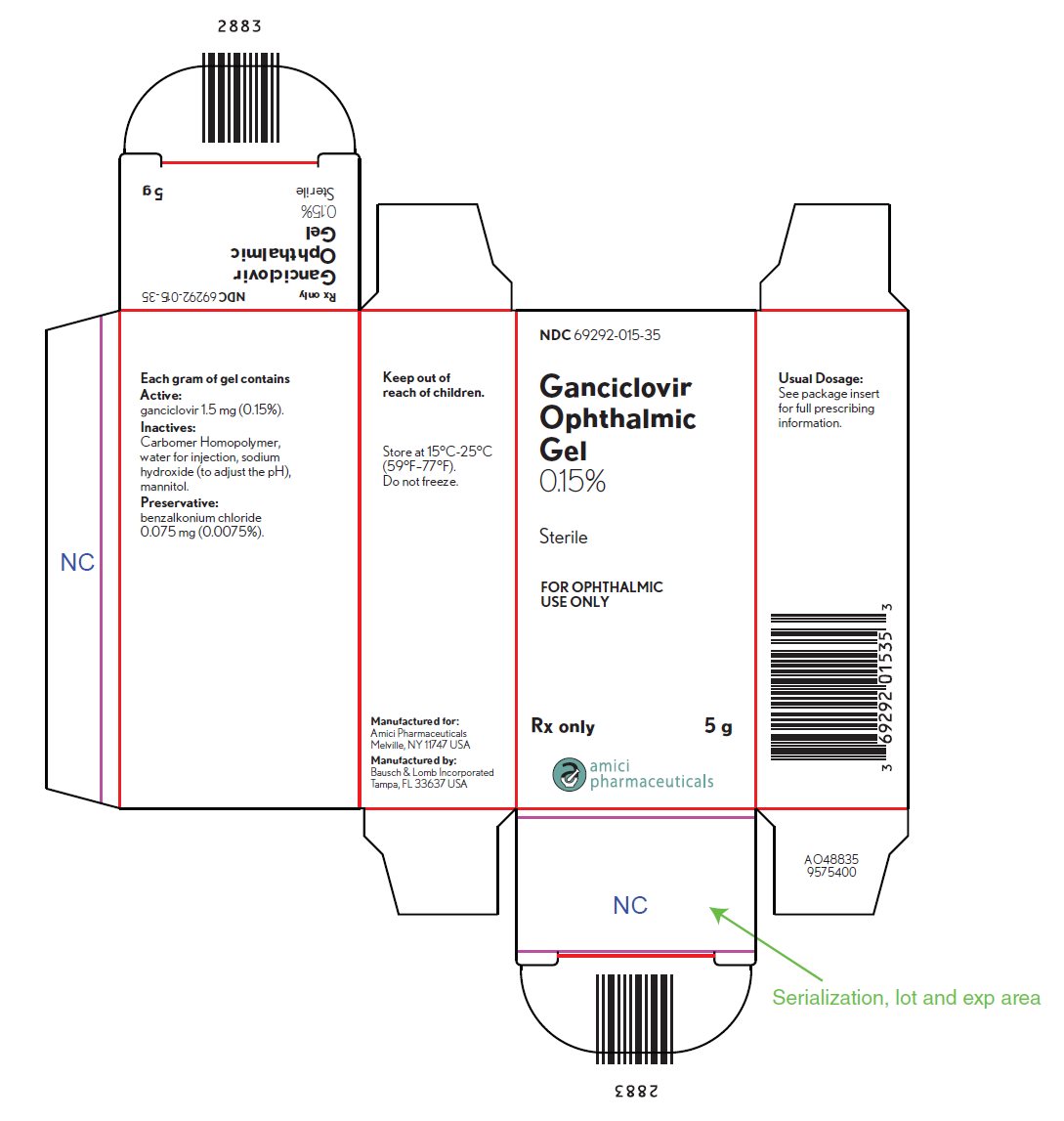
NDC 69292-015-35
Ganciclovir
Ophthalmic
Gel
0.15%
Sterile
FOR OPHTHALMIC
USE ONLY
Rx only
5 g
amici
pharmaceuticals









