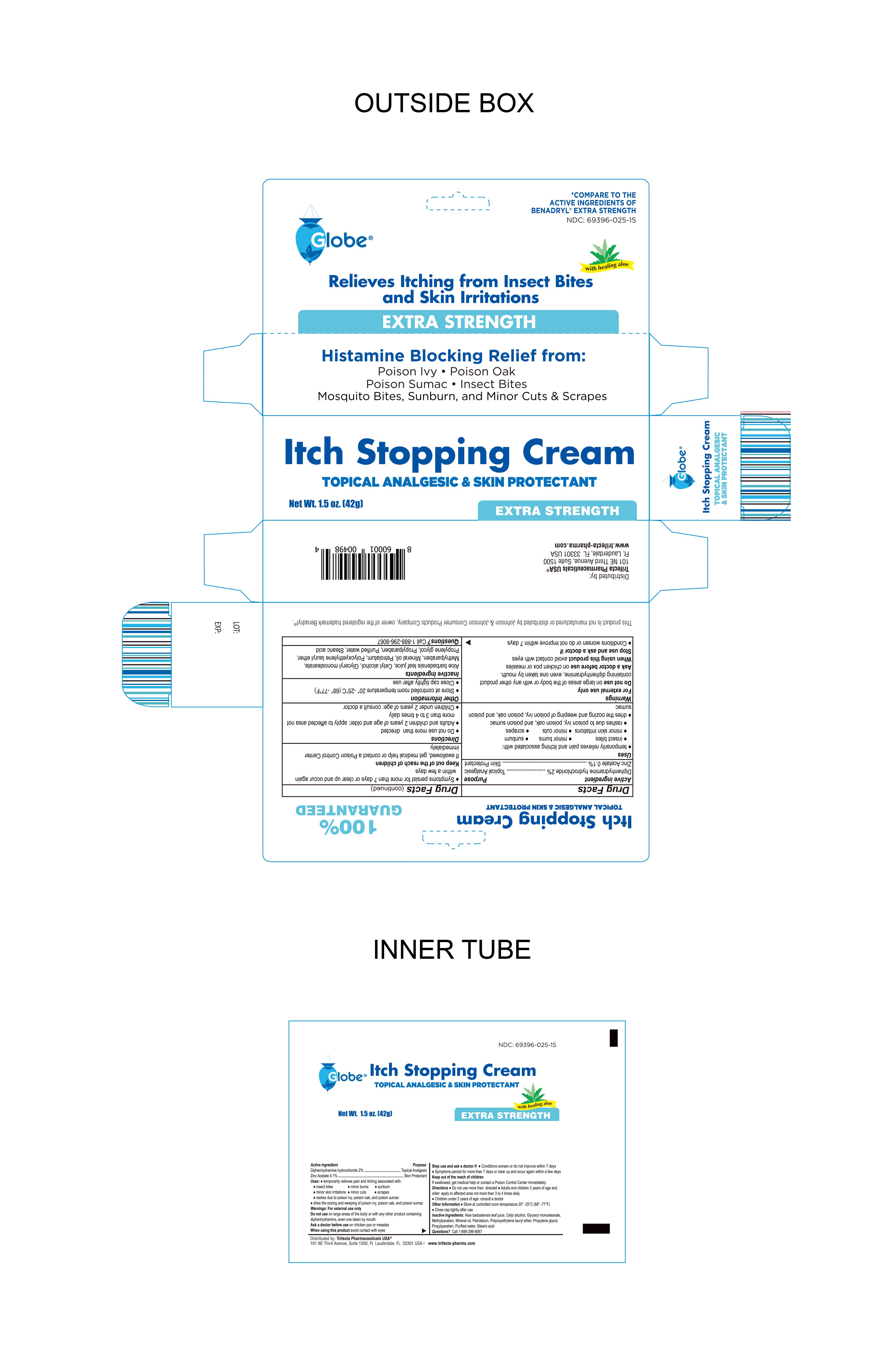
NDC Code(s) : 69396-025-05, 69396-025-15
Packager : Trifecta Pharmaceuticals USA, LLC.
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Anti-Itch CreamDiphenhydramine hydrochloride 2%, zinc acetate 0.1% CREAM | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Trifecta Pharmaceuticals USA, LLC.(079424163) |
PRINCIPAL DISPLAY PANEL