NDC Code(s) : 69539-093-30, 69539-093-90, 69539-093-05, 69539-093-99, 69539-094-30, 69539-094-90, 69539-094-05, 69539-094-99, 69539-095-30, 69539-095-90, 69539-095-05, 69539-095-99, 69539-096-30, 69539-096-90, 69539-096-05, 69539-096-99
Packager : MSN LABORATORIES PRIVATE LIMITED
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Atorvastatin calcium Atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Atorvastatin calcium Atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Atorvastatin calcium Atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Atorvastatin calcium Atorvastatin calcium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - MSN LABORATORIES PRIVATE LIMITED(650786952) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| MSN LABORATORIES PRIVATE LIMITED | 650786952 | ANALYSIS(69539-093, 69539-094, 69539-095, 69539-096), MANUFACTURE(69539-093, 69539-094, 69539-095, 69539-096) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| MSN Pharmaceuticals Inc. | 079229051 | ANALYSIS(69539-093, 69539-094, 69539-095, 69539-096), MANUFACTURE(69539-093, 69539-094, 69539-095, 69539-096) | |
PRINCIPAL DISPLAY PANEL
10mg-30s-container-label
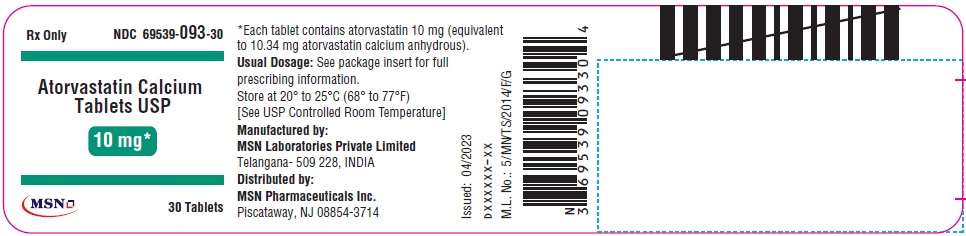
10mg-90s-container-label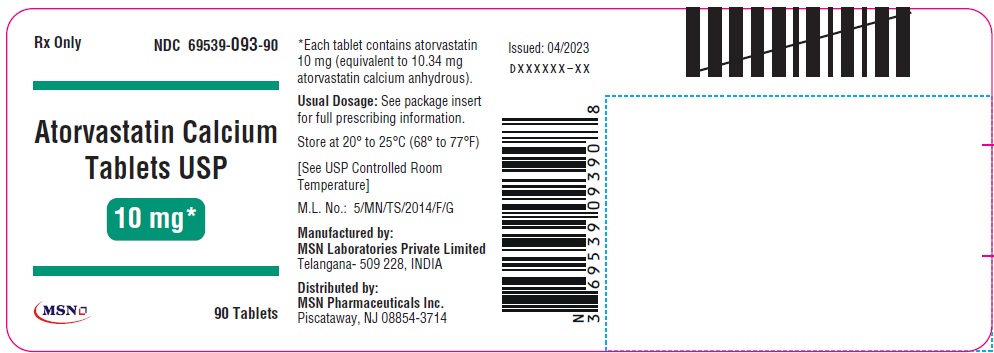
10mg-500s-container-label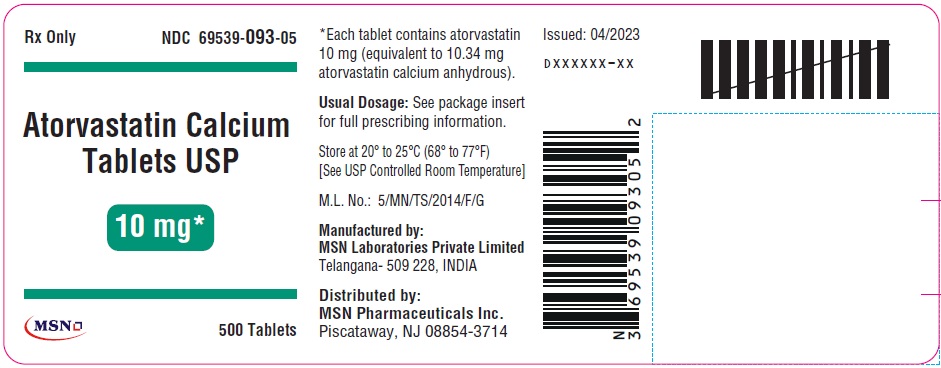
10mg-1000s-container-label
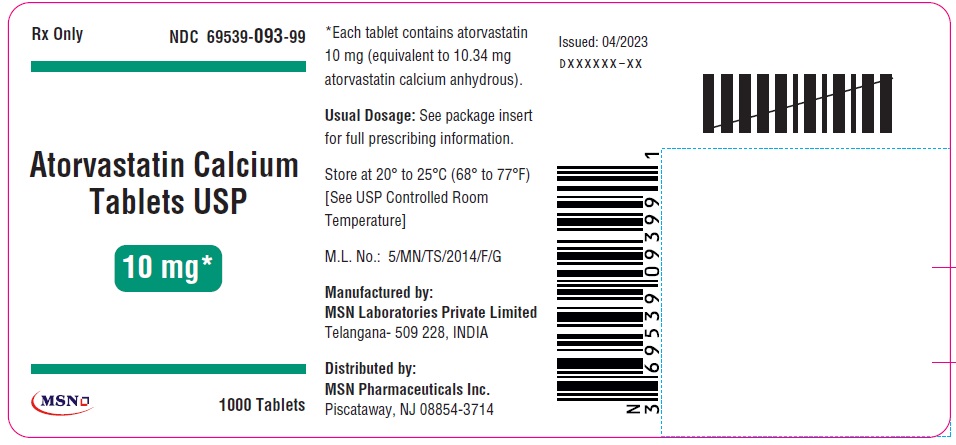
20mg-30s-container-label
20mg-90s-container-label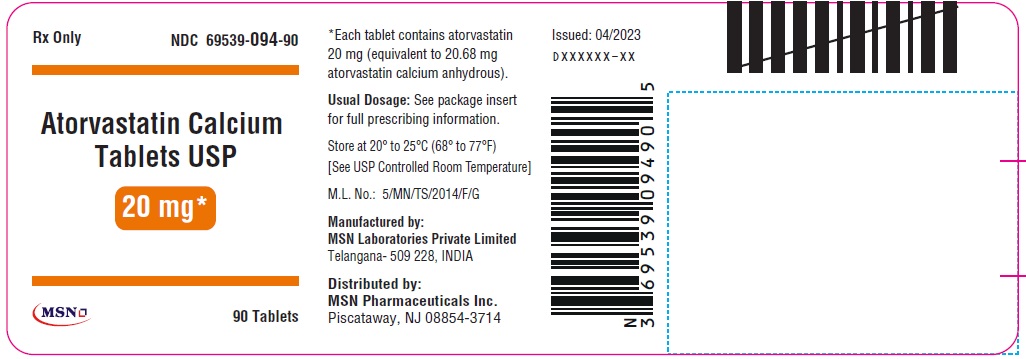
20mg-500s-container-label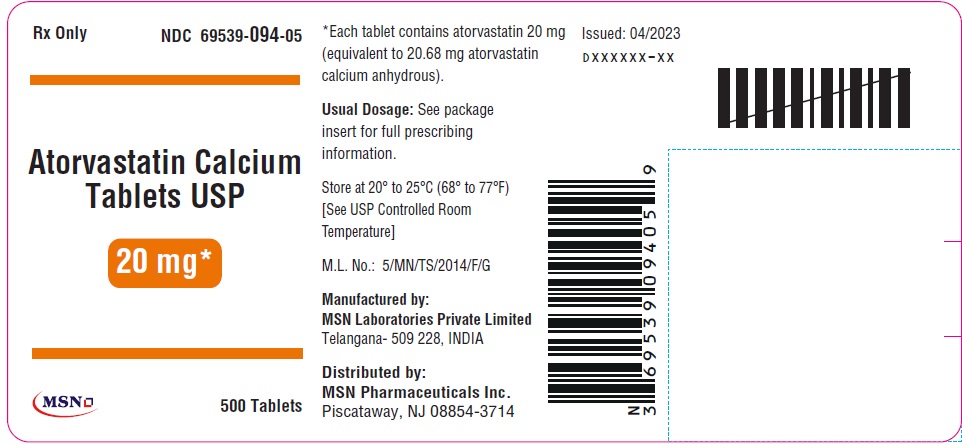
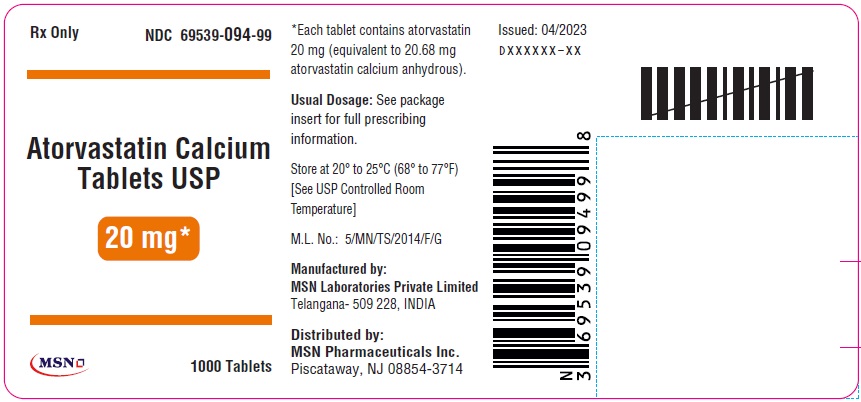
20mg-1000s-container-label
40mg-30s-container-label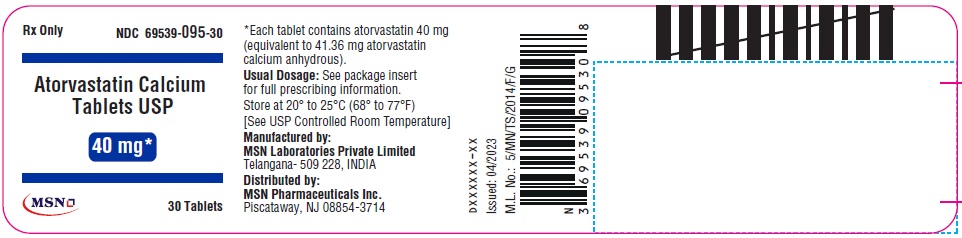
40mg-90s-container-label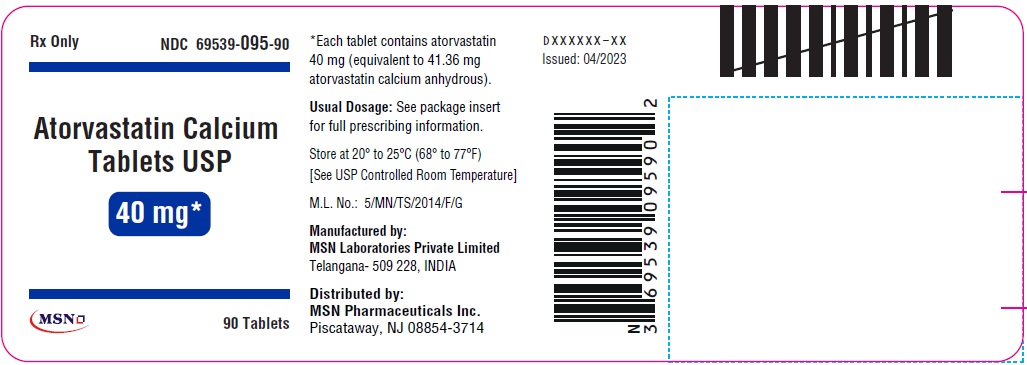
40mg-500s-container-label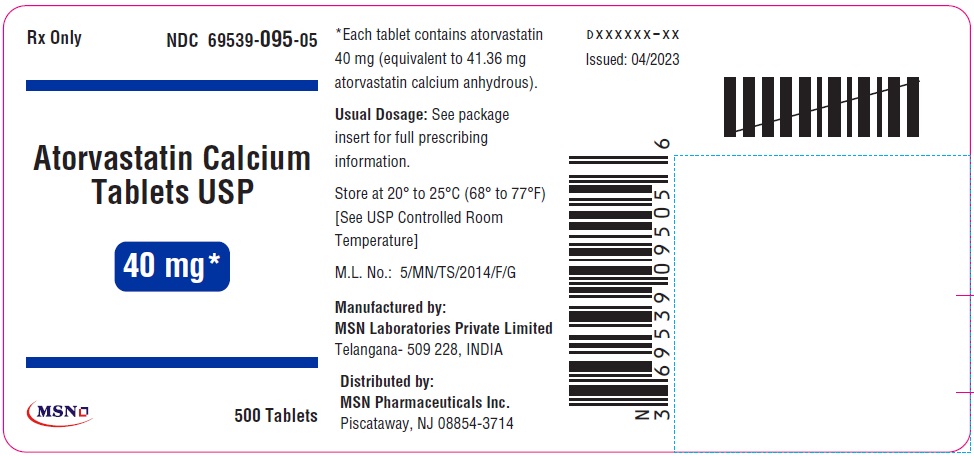
40mg-1000s-container-label
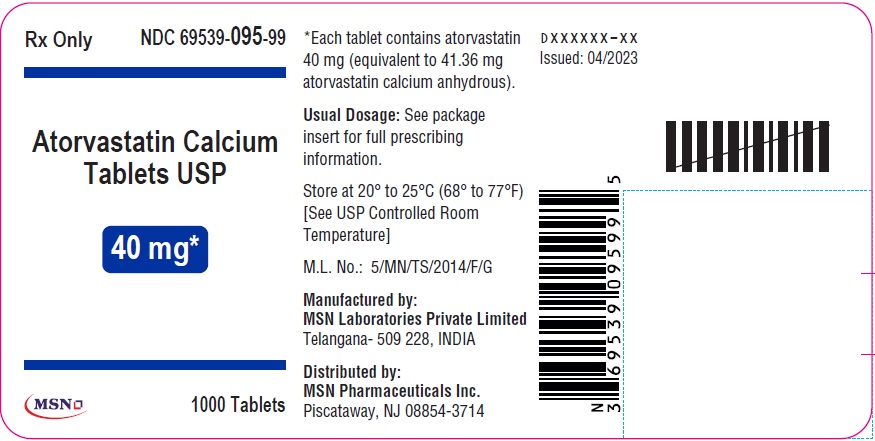
80mg-30s-container-label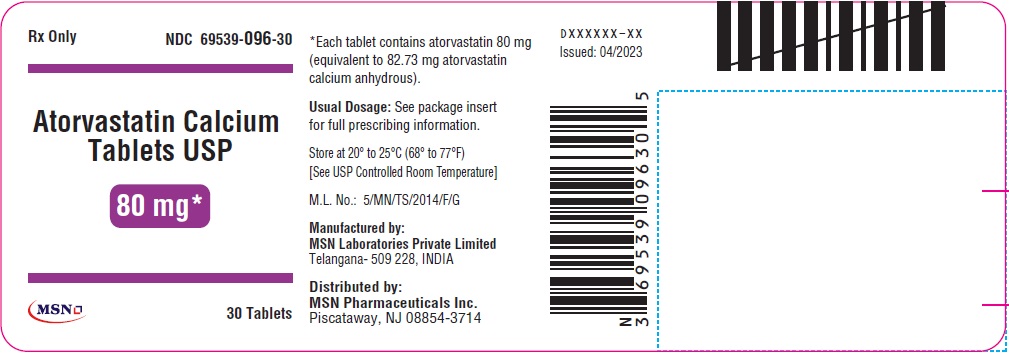
80mg-90s-container-label
80mg-500s-container-label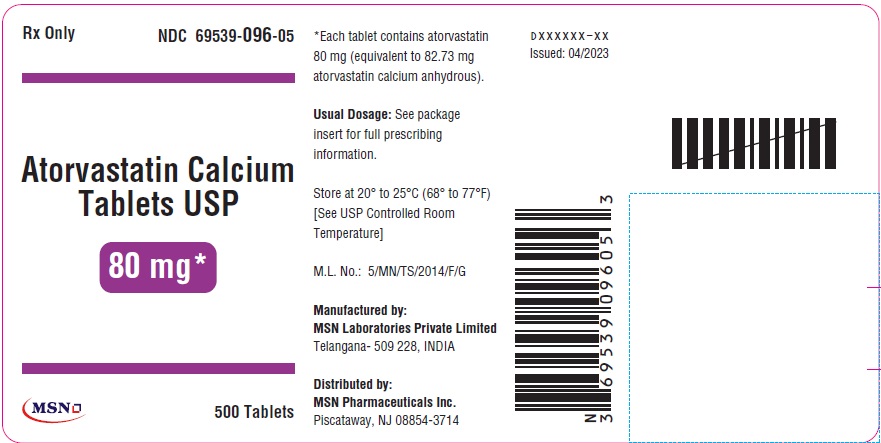
80mg-1000s-container-label










