NDC Code(s) : 69680-112-01, 69680-112-25, 69680-112-10, 69680-113-10, 69680-113-99, 69680-121-05, 69680-121-30, 69680-121-10
Packager : Vitruvias Therapeutics
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CyanocobalaminCyanocobalamin INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CyanocobalaminCyanocobalamin INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CyanocobalaminCyanocobalamin INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Vitruvias Therapeutics(079200795) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| UBI Pharma Inc. | 658871159 | MANUFACTURE(69680-112, 69680-113, 69680-121), PACK(69680-112, 69680-113, 69680-121), ANALYSIS(69680-112, 69680-113, 69680-121) | |
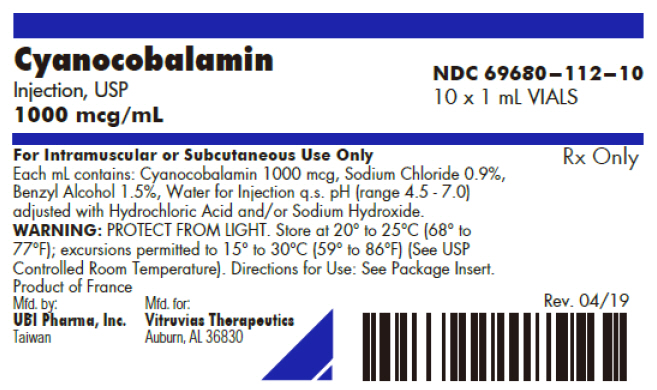
PRINCIPAL DISPLAY PANEL
Cyanocobalamin
Injection, USP
1000 mcg/mL
NDC 69680–112–10
10 x 1 mL VIALS
For Intramuscular or Subcutaneous Use Only
Each mL contains: Cyanocobalamin 1000 mcg, Sodium Chloride 0.9%,
Benzyl Alcohol 1.5%, Water for Injection q.s. pH (range 4.5 - 7.0)
adjusted with Hydrochloric Acid and/or Sodium Hydroxide.
WARNING: PROTECT FROM LIGHT. Store at 20° to 25°C (68° to
77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP
Controlled Room Temperature). Directions for Use: See Package Insert.
Rx Only
Product of France
Mfd. by:
UBI Pharma, Inc.
Taiwan
Mfd. for:
Vitruvias Therapeutics
Auburn, AL 36830
Rev. 04/19
PRINCIPAL DISPLAY PANEL
Cyanocobalamin
Injection, USP
10,000 mcg/10 mL (1,000 mcg/mL)
NDC 69680–113–99
10 x 10 mL
MULTIPLE DOSE VIALS
For Intramuscular or Subcutaneous Use Only
Each mL contains: Cyanocobalamin 1000 mcg, Benzyl Alcohol 1.5%,
Sodium Chloride 0.9%, Water for Injection q.s. pH (range 4.5 - 7.0)
adjusted with Hydrochloric Acid and/or Sodium Hydroxide.
WARNING: PROTECT FROM LIGHT. Store at 20° to 25°C (68° to 77°F);
excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled
Room Temperature).
Directions for Use: See Package Insert.
Product of France
Rx Only
Rev. 06/18
Mfd. by:
UBI Pharma, Inc.
Taiwan
Mfd. for:
Vitruvias Therapeutics
Auburn, AL 36830

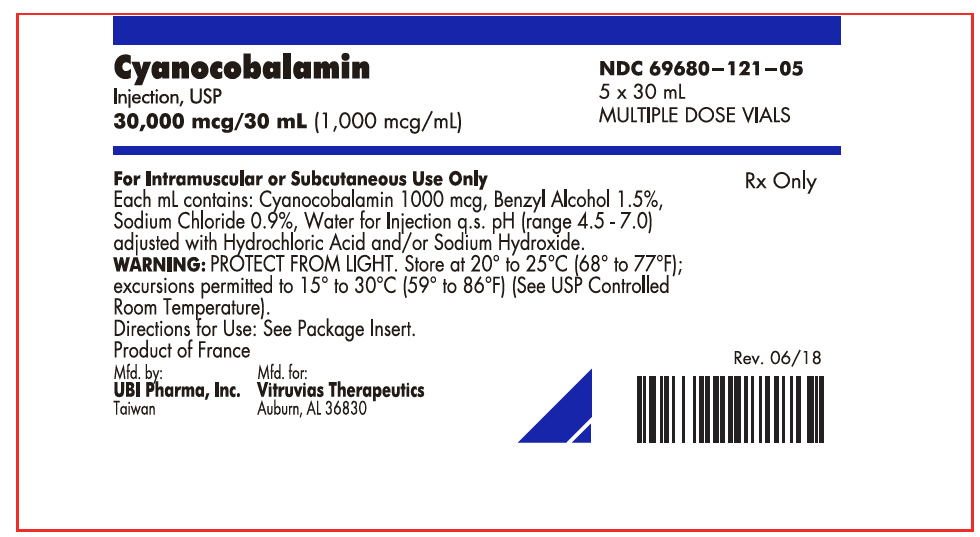
PRINCIPAL DISPLAY PANEL
Cyanocobalamin
Injection, USP
30,000 mcg/30 mL (1,000 mcg/mL)
NDC 69680–121–05
5 x 30 mL
MULTIPLE DOSE VIALS
For Intramuscular or Subcutaneous Use Only
Each mL contains: Cyanocobalamin 1000 mcg, Benzyl Alcohol 1.5%,
Sodium Chloride 0.9%, Water for Injection q.s. pH (range 4.5 - 7.0)
adjusted with Hydrochloric Acid and/or Sodium Hydroxide.
WARNING: PROTECT FROM LIGHT. Store at 20° to 25°C (68° to 77°F);
excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled
Room Temperature).
Directions for Use: See Package Insert.
Product of France
Rx Only
Rev. 06/18
Mfd. by:
UBI Pharma, Inc.
Taiwan
Mfd. for:
Vitruvias Therapeutics
Auburn, AL 36830