NDC Code(s) : 69842-207-92
Packager : CVS Pharmacy
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CVS Decolorized IodineAlcohol LIQUID | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| LABELER - CVS Pharmacy(062312574) |
| REGISTRANT - Pharma Nobis, LLC(118564114) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pharma Nobis, LLC | 118564114 | analysis(69842-207), manufacture(69842-207), pack(69842-207), label(69842-207) | |
PRINCIPAL DISPLAY PANEL

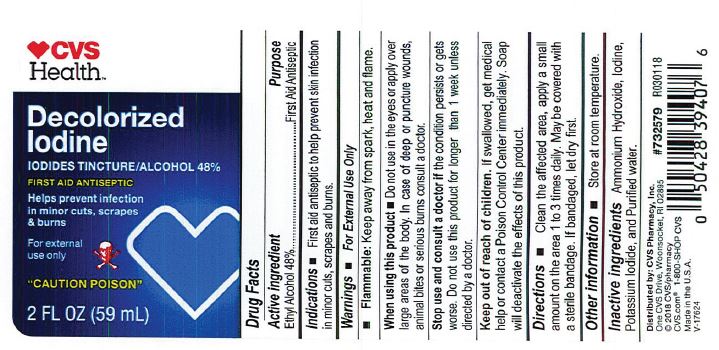 Decolorized
Decolorized
Iodine
First Aid Antiseptic
2 FL OZ (59 mL)










