NDC Code(s) : 69842-921-49
Packager : CVS Pharmacy
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CVS Lidocaine Lidocaine HCl CREAM | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| LABELER - CVS Pharmacy(062312574) |
PRINCIPAL DISPLAY PANEL
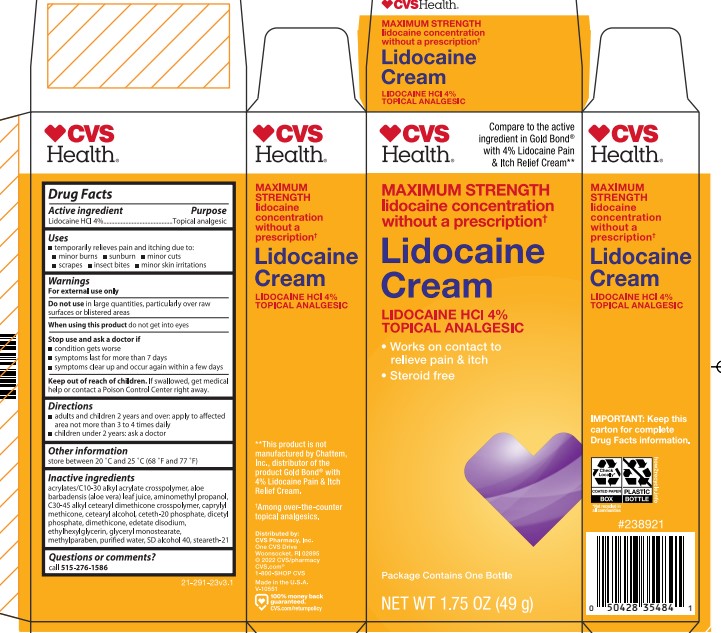 CVS Health
CVS Health
Multi- Symptom Formula Works On Contact to relieve Pain & Itch From:
Minor Burns & cuts
Minor Skin Irritaions
Insect bites
Sunburns
Maximum Strength Lidocaine Cream Lidocaine HCL 4 %- Topical Analgesic
Steroid free, Works on Contact









