NDC Code(s) : 69844-008-01, 69844-008-02, 69844-008-03, 69844-009-01, 69844-009-02, 69844-009-03
Packager : Graviti Pharmaceuticals Private Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Fenofibrate fenofibrate TABLET | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Fenofibrate fenofibrate TABLET | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LABELER - Graviti Pharmaceuticals Private Limited(650884781) |
| REGISTRANT - Graviti Pharmaceuticals Private Limited(650884781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Graviti Pharmaceuticals Private Limited | 650884781 | manufacture(69844-008, 69844-009), analysis(69844-008, 69844-009) | |
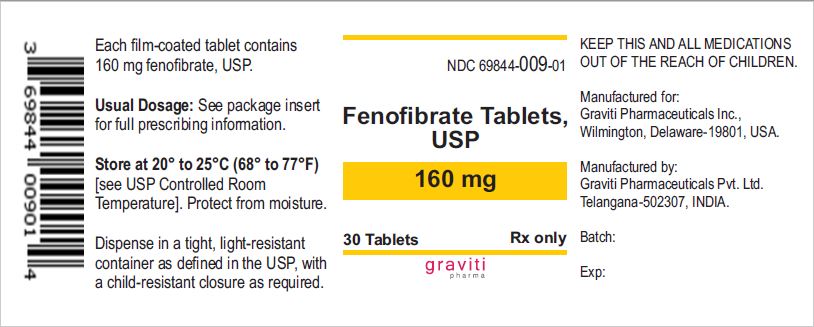
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 54 mg Tablet Bottle Label
NDC 69844-008-01
30 Tablets
Rx only
Fenofibrate Tablets USP
54 mg
Manufactured for Graviti Pharmaceuticals Inc.
Made in India.
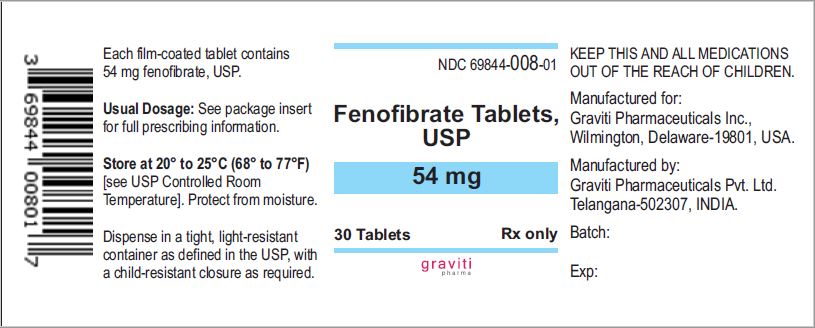
PRINCIPAL DISPLAY PANEL - 160 mg Tablet Bottle Label
NDC 69844-009-01
30 Tablets
Rx only
Fenofibrate Tablets USP
160 mg
Manufactured for Graviti Pharmaceuticals Inc.
Made in India.