NDC Code(s) : 70121-1244-1, 70121-1244-7
Packager : Amneal Pharmaceuticals LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BusulfanBusulfan INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Amneal Pharmaceuticals LLC(827748190) |
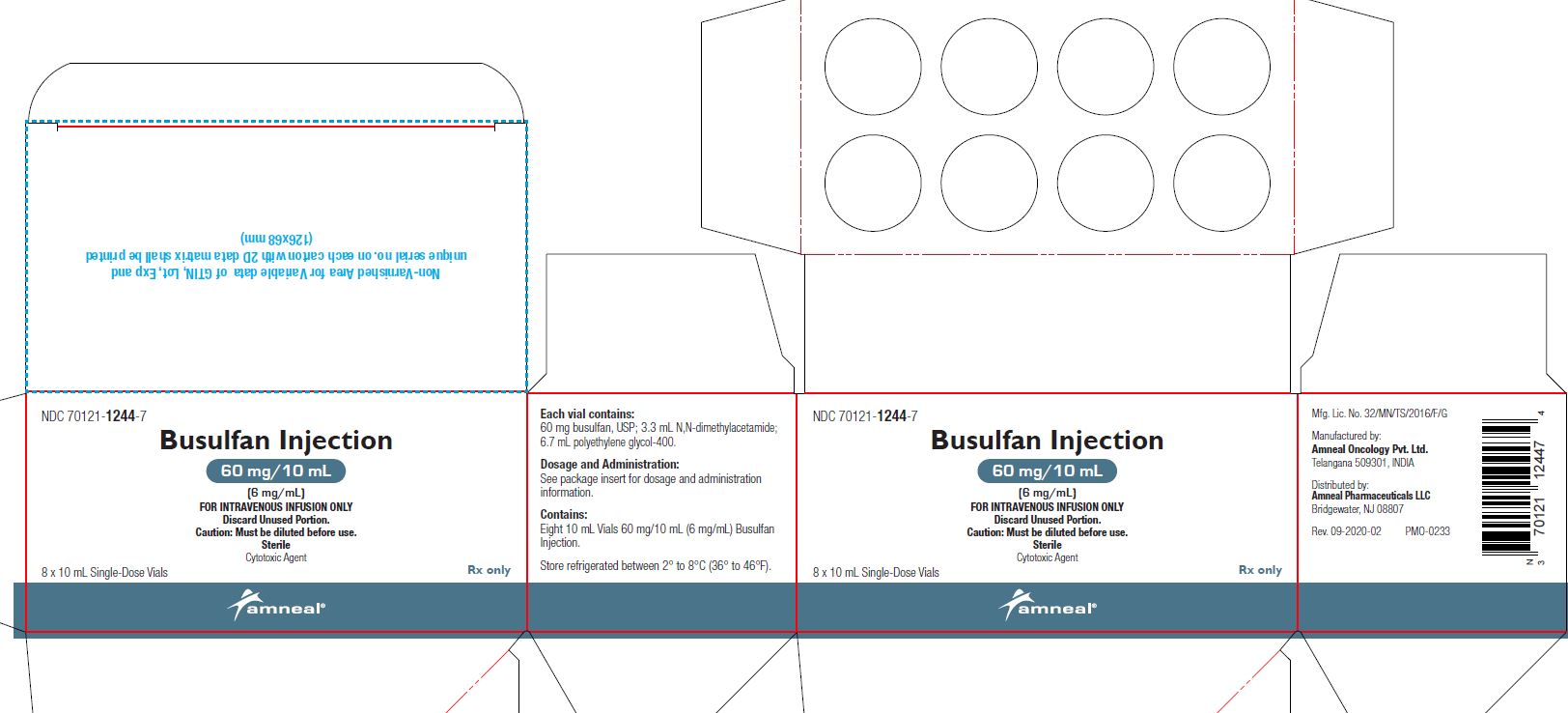
PRINCIPAL DISPLAY PANEL
NDC 70121-1244-1
Strength: 60 mg/10 mL (6 mg/mL)
Rx only
Vial Label
Amneal Pharmaceuticals LLC

NDC 70121-1244-7
Strength: 60 mg/10 mL (6 mg/mL)
Rx only
Carton Label
Amneal Pharmaceuticals LLC