NDC Code(s) : 70253-235-08, 70253-235-15
Packager : NASH-FINCH COMPANY
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Pain Reliever PMAcetaminophen and Diphenhydramine HCl TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
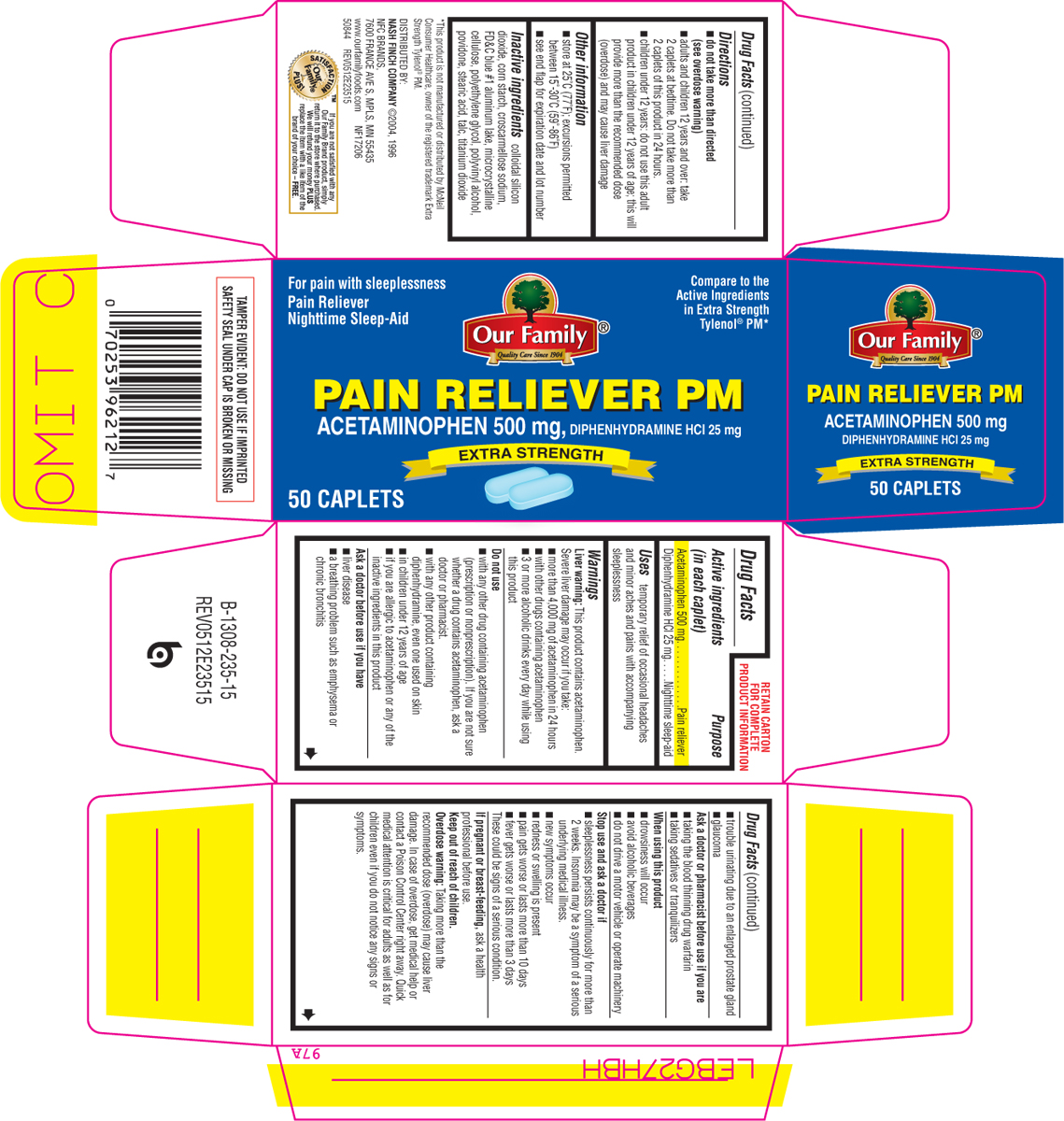
PRINCIPAL DISPLAY PANEL
For pain with sleeplessness
Pain Reliever
Nighttime Sleep-Aid
Our Family®
Quality Care Since 1904
Compare to the
Active Ingredients
in Extra Strength
Tylenol® PM*
PAIN RELIEVER PM
ACETAMINOPHEN 500 mg, DIPHENHYDRAMINE HCl 25 mg
EXTRA STRENGTH
50 CAPLETS
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Extra Strength Tylenol® PM.
DISTRIBUTED BY:
NASH FINCH COMPANY ©2004,1996
NFC BRANDS,
7600 FRANCE AVE S, MPLS, MN 55435
www.ourfamilyfoods.com NF17206
50844 REV0512E23515
 OurFamily44-235
OurFamily44-235









