NDC Code(s) : 70461-002-11, 70461-002-01
Packager : Seqirus Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| FLUADA/Singapore/GP1908/2015 IVR-180 (H1N1) (an A/Michigan/45/2015 (H1N1)pdm09-like virus), A/Hong Kong/4801/2014, NYMC X-263B (H3N2) (an A/Hong Kong/4801/2014-like virus), and B/Brisbane/60/2008, wild type (a B/Brisbane/60/2008-like virus) INJECTION, SUSPENSION | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
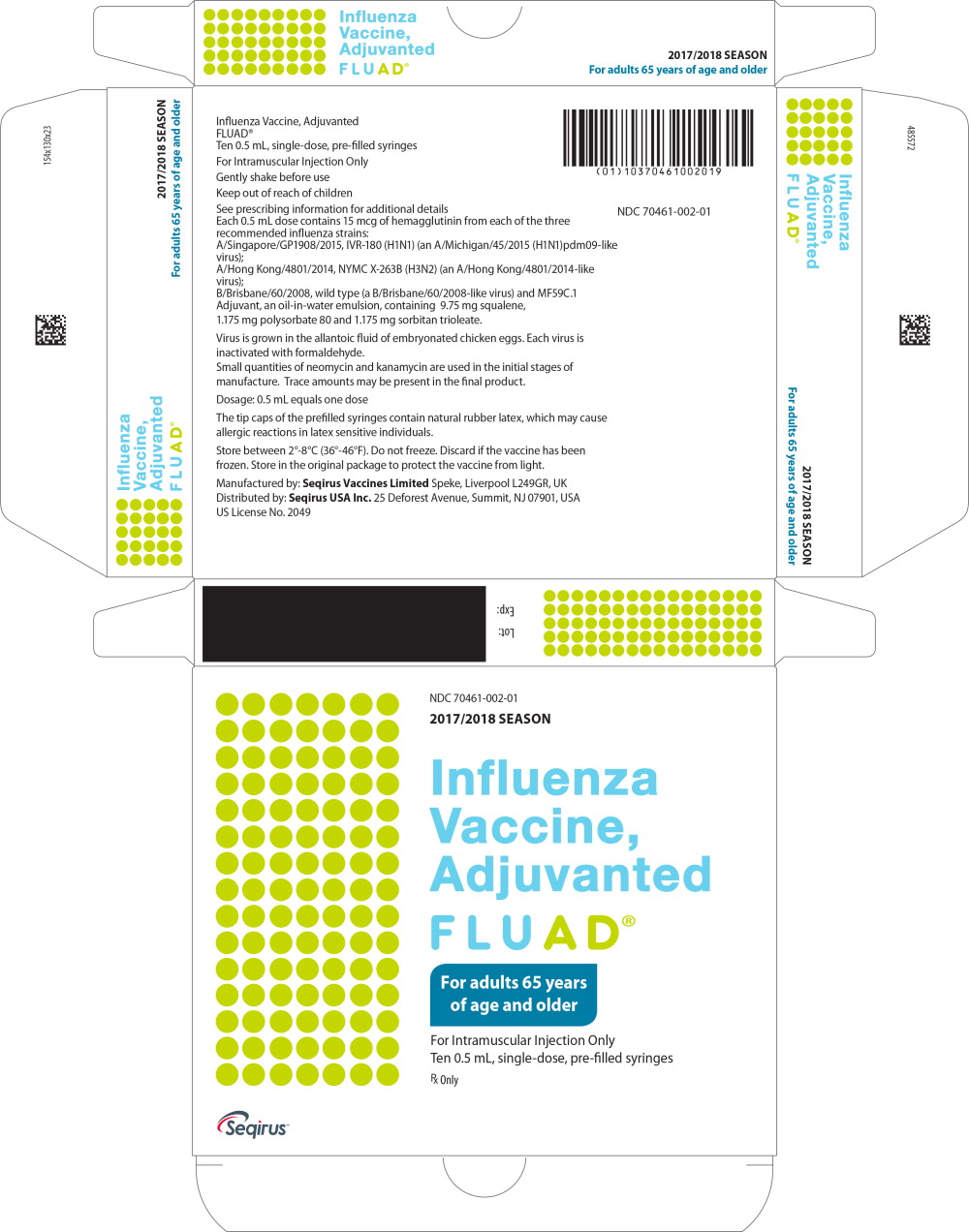
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Fluad Carton Label
NDC 70641-002-01
2017/2018 SEASON
Influenza
Vaccine,
Adjuvanted
FLUAD
®
For adults 65 years
of age and older
For Intramuscular Injection Only
Ten 0.5 mL, single-dose, pre-filled syringes
Rx Only
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Fluad Label
Influenza Vaccine, Adjuvanted
FLUAD® 2017/2018 season










