NDC Code(s) : 70461-201-11, 70461-201-01, 70461-301-12, 70461-301-10
Packager : Seqirus, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| FLUCELVAX QUADRIVALENT (PREFILLED SYRINGE)A/Singapore/GP1908/2015 IVR-180 (H1N1) (an A/Michigan/45/2015-like virus), A/Singapore/GP2050/2015 (H3N2) (an A/Hong Kong/4801/2014 - like virus), B/Utah/9/2014 (a B/Phuket/3073/2013-like virus), B/Hong Kong/259/2010 (a B/Brisbane/60/08-like virus) INJECTION, SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FLUCELVAX QUADRIVALENT (MULTI-DOSE VIAL)A/Singapore/GP1908/2015 IVR-180 (H1N1) (an A/Michigan/45/2015-like virus), A/Singapore/GP2050/2015 (H3N2) (an A/Hong Kong/4801/2014 - like virus), B/Utah/9/2014 (a B/Phuket/3073/2013-like virus), B/Hong Kong/259/2010 (a B/Brisbane/60/08-like virus) INJECTION, SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
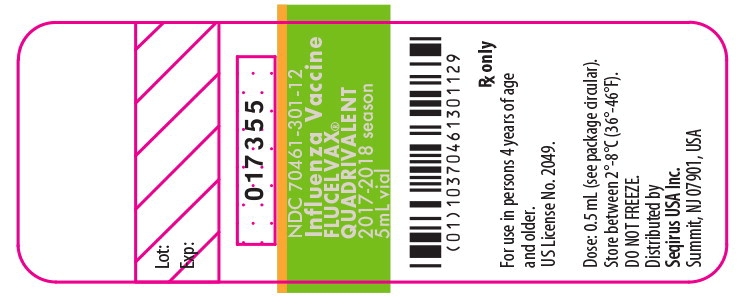
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 0.5 mL Carton Label
NDC 70461-201-01
For use in persons 4 years of age and older
For intramuscular Injection only
Ten 0.5 mL, single-dose, pre-filled syringes
Rx Only
Influenza Vaccine
FLUCELVAX
®
QUADRIVALENT
2017/2018 SEASON
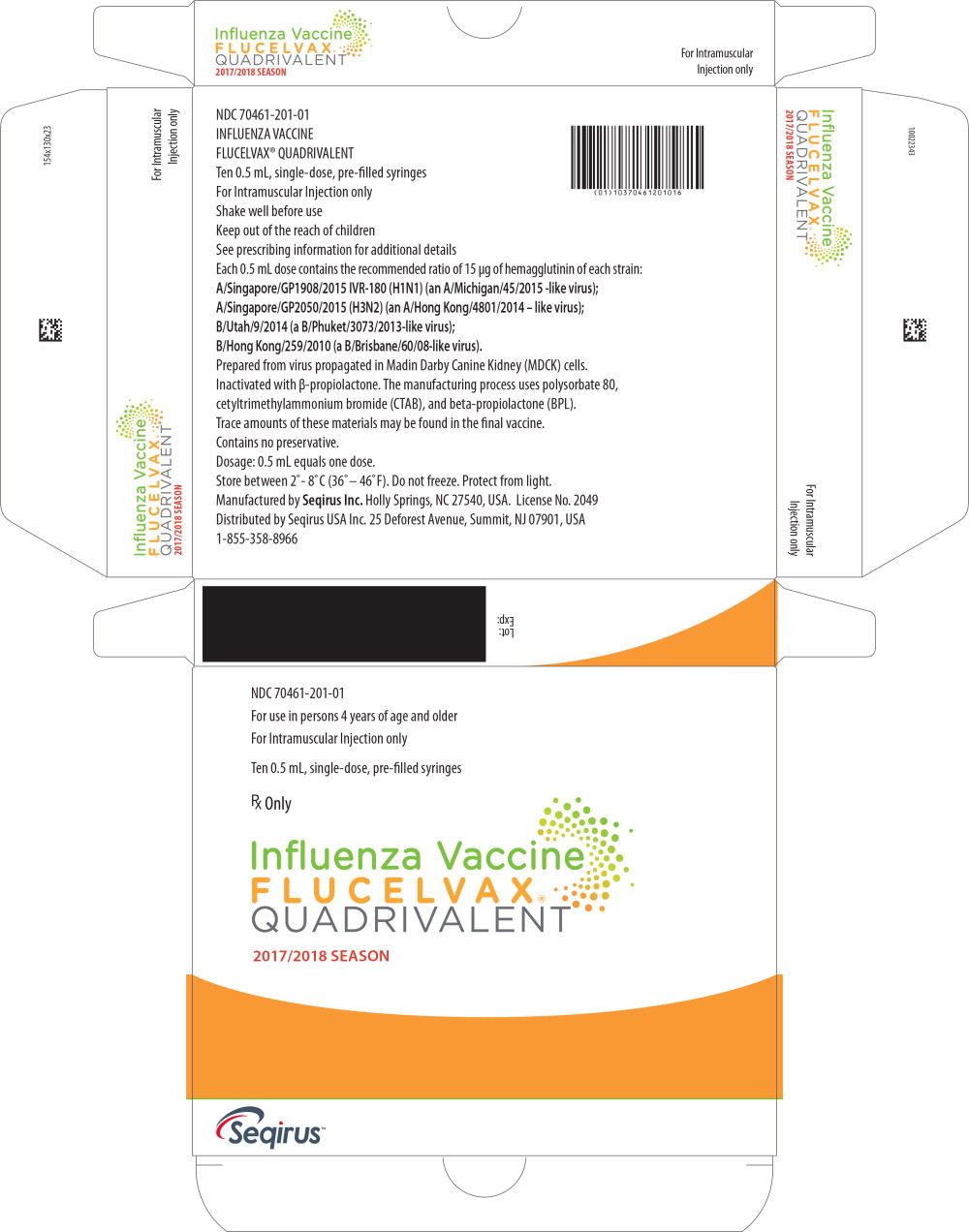
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 0.5 mL Label
Influenza Vaccine
FLUCELVAX
®
QUADRIVALENT
2017/2018 season
For Intramuscular Injection Only
NDC 70461-201-11
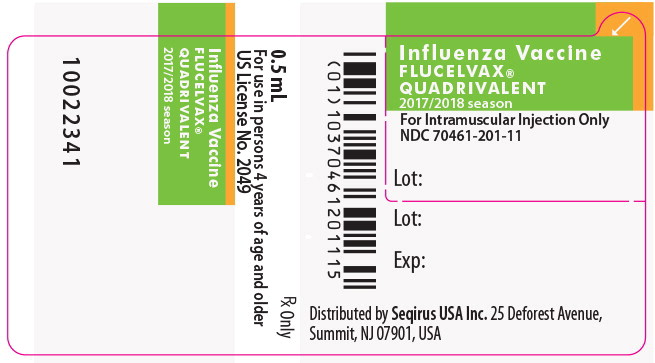
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 5 mL Carton Label
Seqirus™
NDC 70461-301-10
5 mL vial
Rx Only
For use in persons 4 years of age and older
For Intramuscular Injection Only
Influenza Vaccine
FLUCELVAX
®
QUADRIVALENT
2017/2018 SEASON
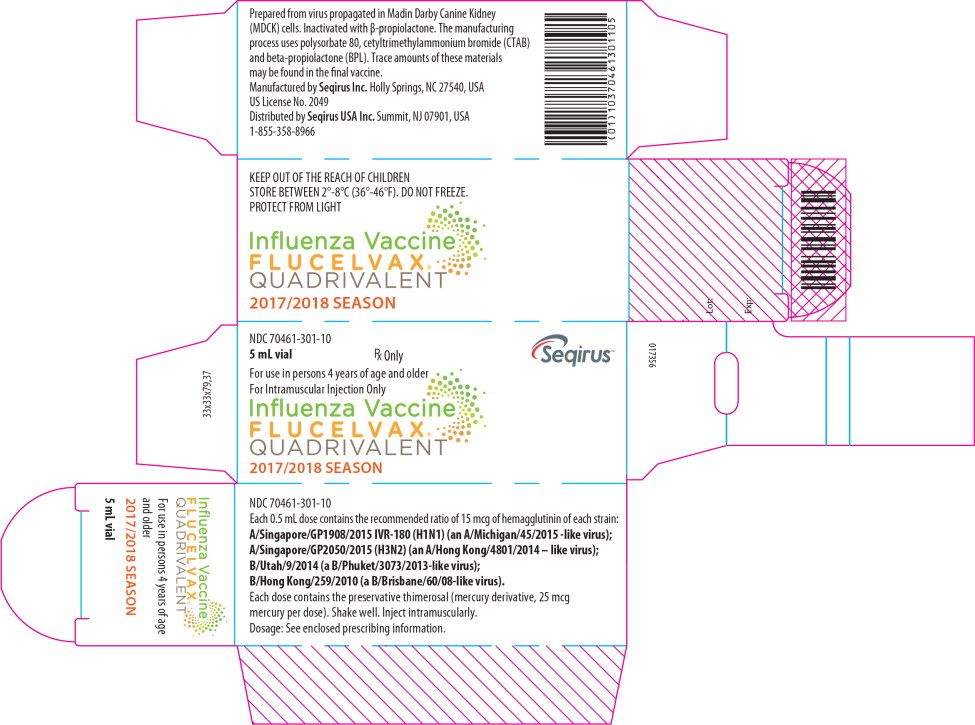
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 5 mL Label
NDC 70461-301-12
Influenza Vaccine
FLUCELVAX
®
QUADRIVALENT
2017-2018 season
5mL vial
Rx only