NDC Code(s) : 70677-0060-1
Packager : STRATEGIC SOURCING SERVICES, LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Anti-DiarrhealLOPERAMIDE HYDROCHLORIDE CAPSULE, LIQUID FILLED | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - STRATEGIC SOURCING SERVICES, LLC(116956644) |
| REGISTRANT - Bionpharma Inc.(079637826) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Patheon Softgels Inc. | 002193829 | manufacture(70677-0060) | |
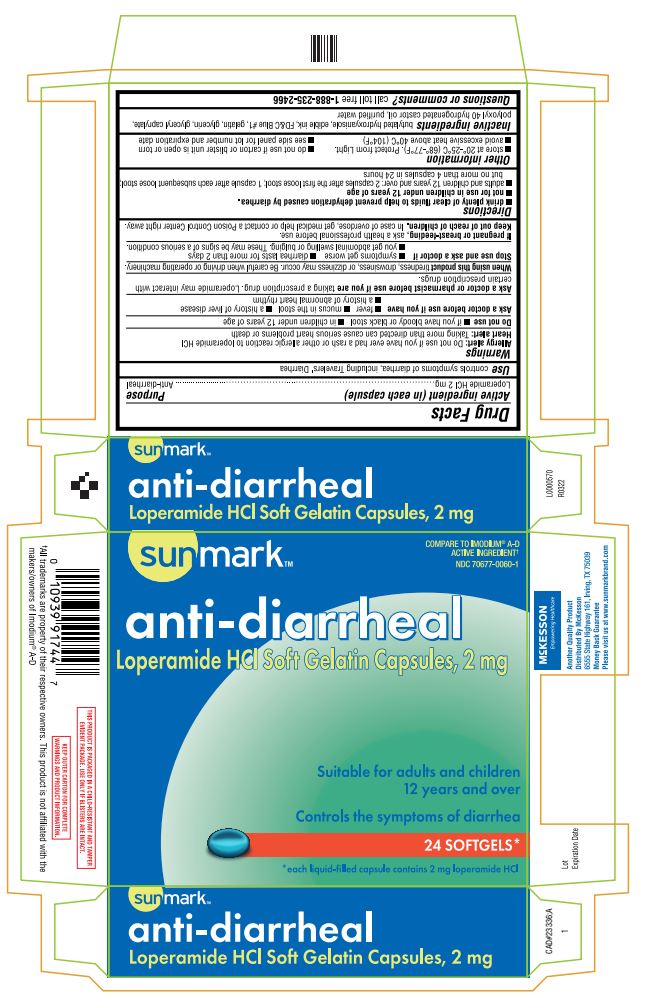
PRINCIPAL DISPLAY PANEL
sunmark TM
COMPARE TO IMODIUM ® A-D
ACTIVE INGREDIENT†
NDC 70677-0060-1
anti-diarrheal
Loperamide HCl Soft Gelatin Capsules, 2 mg
Suitable for adults and children
12 years and over
Controls the symptoms of diarrhea
24 SOFTGELS*
*each liquid-filled capsule contains 2 mg loperamide HCl