NDC Code(s) : 70748-299-01
Packager : Lupin Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ATOVAQUONE ATOVAQUONE SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Lupin Pharmaceuticals, Inc.(089153071) |
| REGISTRANT - Hetero Labs Limited Unit-III(676162024) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Hetero Labs Limited Unit-III | 676162024 | MANUFACTURE(70748-299), PACK(70748-299) | |
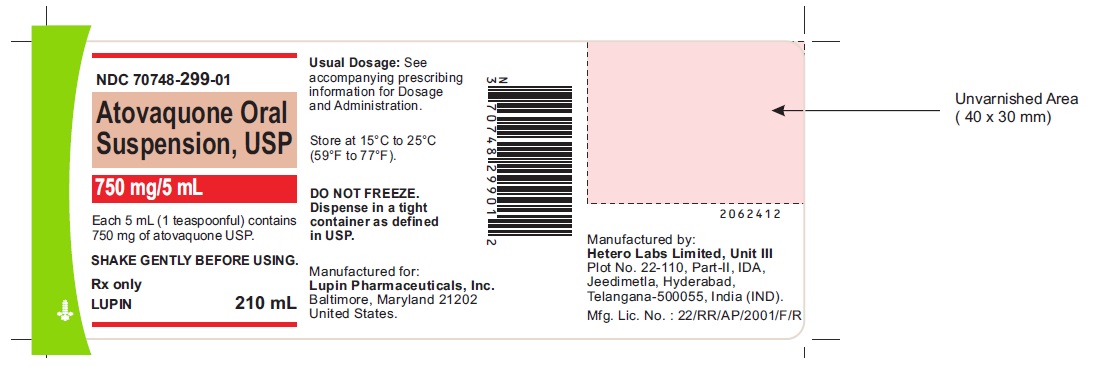
PRINCIPAL DISPLAY PANEL
ATOVAQUONE ORAL SUSPENSION USP
Rx Only
210 mL
NDC 70748-299-01
750 mg/5 mL
Each 5 mL (1 teaspoonful) contains 750 mg of atovaquone USP.
SHAKE GENTLY BEFORE USING .
See accompanying prescribing information for Dosage and Administration
Store at 15 ºC to 25ºC (59 ºF to 77ºF)
DO NOT FREEZE. Dispense in a tight container as defined in USP.