NDC Code(s) : 70771-1338-1, 70771-1338-5, 70771-1338-0, 70771-1339-1, 70771-1339-5, 70771-1339-0, 70771-1340-1, 70771-1340-5, 70771-1340-0, 70771-1341-1, 70771-1341-5, 70771-1341-0
Packager : Zydus Lifesciences Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| metoprolol succinate metoprolol succinate TABLET, EXTENDED RELEASE | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| metoprolol succinate metoprolol succinate TABLET, EXTENDED RELEASE | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| metoprolol succinate metoprolol succinate TABLET, EXTENDED RELEASE | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| metoprolol succinate metoprolol succinate TABLET, EXTENDED RELEASE | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LABELER - Zydus Lifesciences Limited(918596198) |
| REGISTRANT - Zydus Lifesciences Limited(863362789) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Zydus Lifesciences Limited | 863362789 | ANALYSIS(70771-1338, 70771-1339, 70771-1340, 70771-1341), MANUFACTURE(70771-1338, 70771-1339, 70771-1340, 70771-1341) | |
PRINCIPAL DISPLAY PANEL
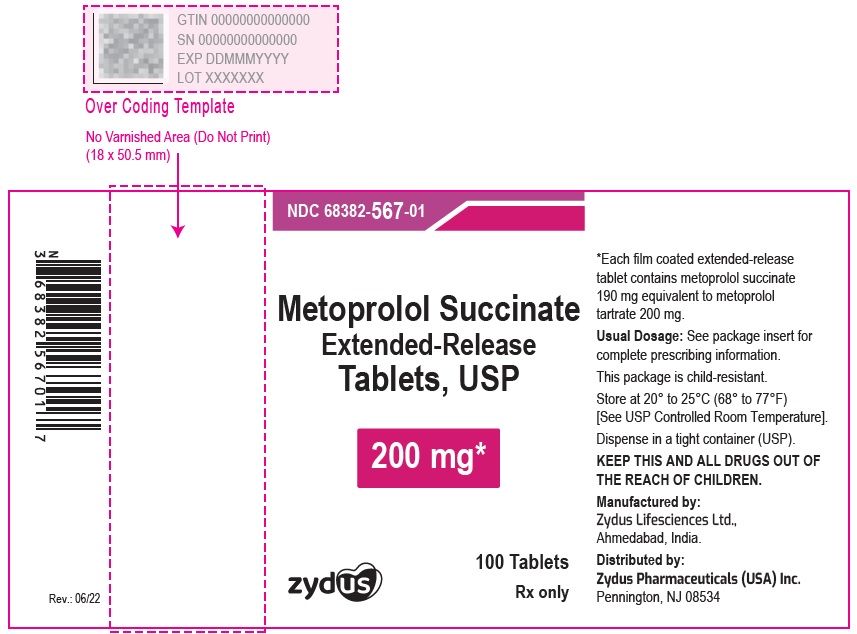
NDC 70771-1338-1 in bottles of 100 Tablets
Metoprolol Succinate Extended-release Tablets USP, 25 mg
Rx only
100 Tablets
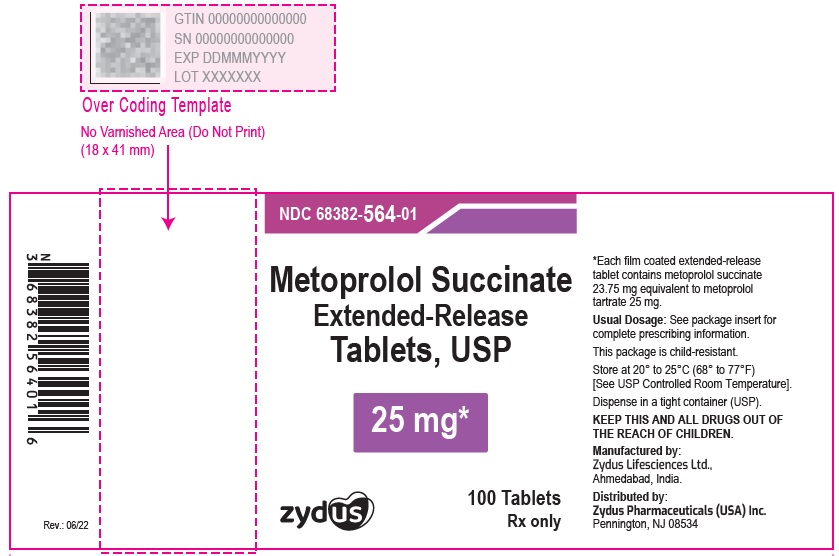
NDC 70771-1339-1 in bottles of 100 Tablets
Metoprolol Succinate Extended-release Tablets USP, 50 mg
Rx only
100 Tablets
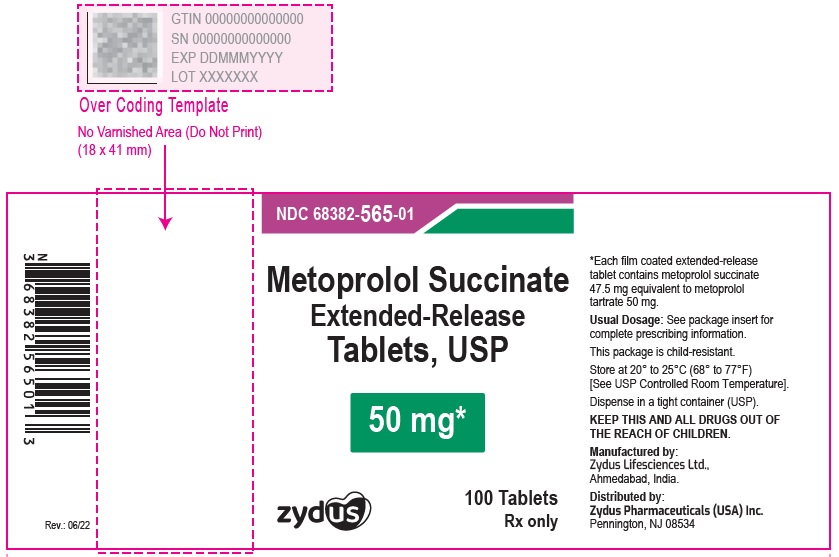
NDC 70771-1340-1 in bottles of 100 Tablets
Metoprolol Succinate Extended-release Tablets USP, 100 mg
Rx only
100 Tablets
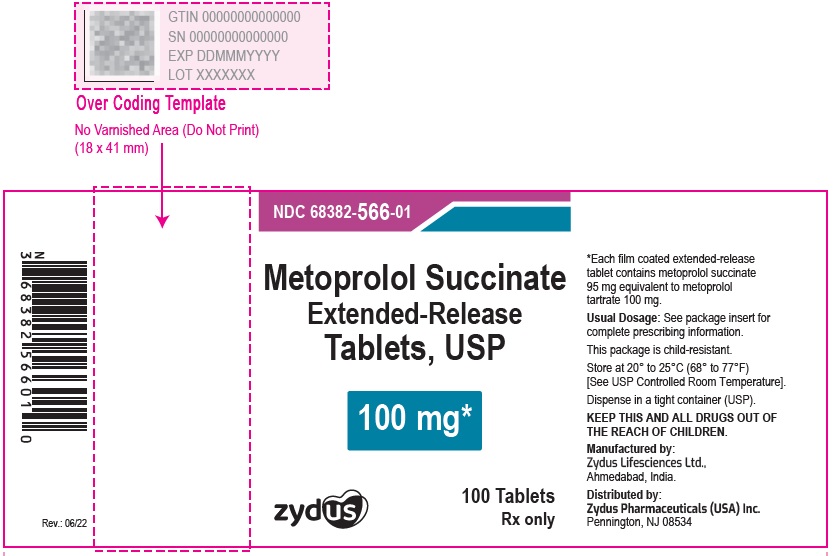
NDC 70771-1341-1 in bottles of 100 Tablets
Metoprolol Succinate Extended-release Tablets USP, 200 mg
Rx only
100 Tablets