NDC Code(s) : 70771-1668-4, 70771-1668-5
Packager : Zydus Lifesciences Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Sildenafil CitrateSildenafil Citrate POWDER, FOR SUSPENSION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Zydus Lifesciences Limited(918596198) |
| REGISTRANT - Zydus Lifesciences Limited(677605858) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Zydus Lifesciences Limited | 677605858 | ANALYSIS(70771-1668), MANUFACTURE(70771-1668) | |
PRINCIPAL DISPLAY PANEL
10 mg/mL Amber Glass Bottle Label
NDC 70771-1668-4
Sildenafil for Oral Suspension
10 mg/mL
Shake Well Before Each Use
For Oral Use Only
Zydus
112 mL following Reconstitution
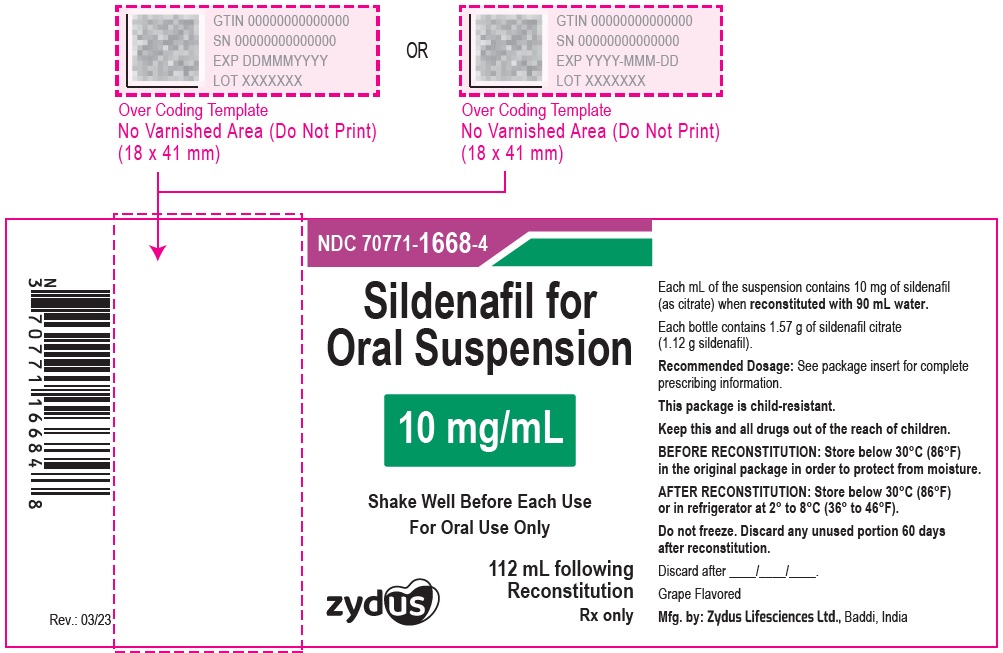
PRINCIPAL DISPLAY PANEL - 10 mg/mL Amber Glass Bottle Carton
NDC 70771-1668-4
Sildenafil for Oral Suspension
10 mg/ml
For Oral Use Only
Shake Well Before Each Use
112 mL following Reconstitution
Grape Flavored
Zydus
Rx only

10 mg/mL HDPE Bottle Label
NDC 70771-1668-5
Sildenafil for Oral Suspension
10 mg/mL
Shake Well Before Each Use
For Oral Use Only
Zydus
112 mL following Reconstitution
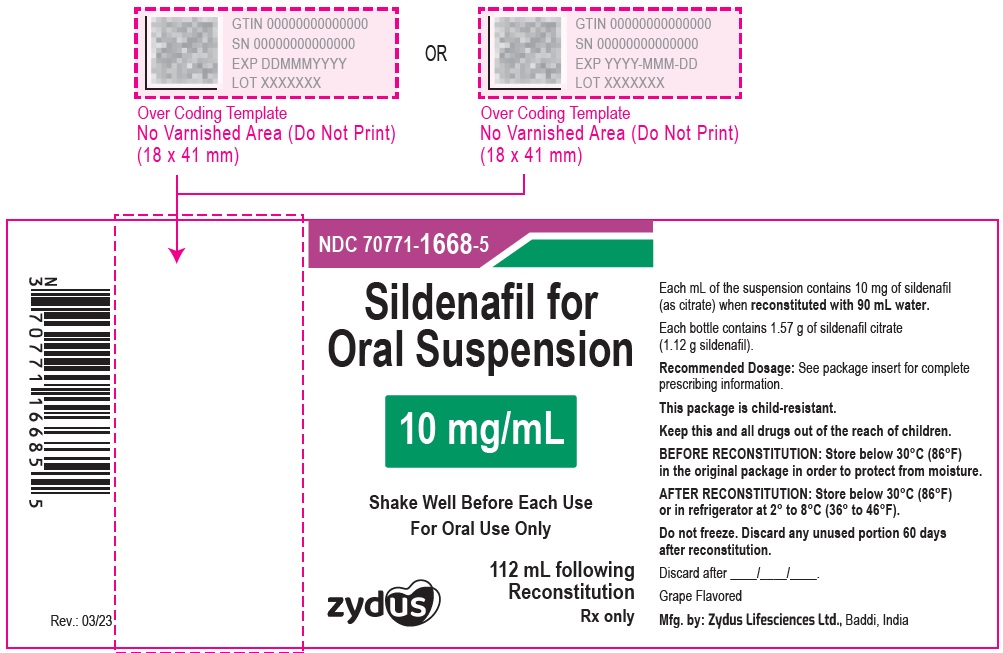
PRINCIPAL DISPLAY PANEL - 10 mg/mL HDPE Bottle Carton
NDC 70771-1668-5
Sildenafil for Oral Suspension
10 mg/ml
For Oral Use Only
Shake Well Before Each Use
112 mL following Reconstitution
Grape Flavored
Zydus
Rx only










